Похожие презентации:
Joint Programs – Health Sciences
1.
Kingdom of Saudi Arabia KingKhalid University
Joint Programs – Health
Sciences
1st Semester, 1440-1441 H
سيعدال اللهدبع نيمأ.د
Dr. AMIN ABDULLAH AL-DOAISS
BIOLOGY
2019
Dr. Amin Al-Doaiss
1
Recommended Textbooks
1)Human Biology, Sylvia S. Mader; 15th Ed, 2017.
2) Biology 6th Ed., Campbell and Reece, 1999
3) Human Biology, Daniel D. Chiras, 2005
BIOLOGY
2019
Dr. Amin Al-Doaiss
2
2.
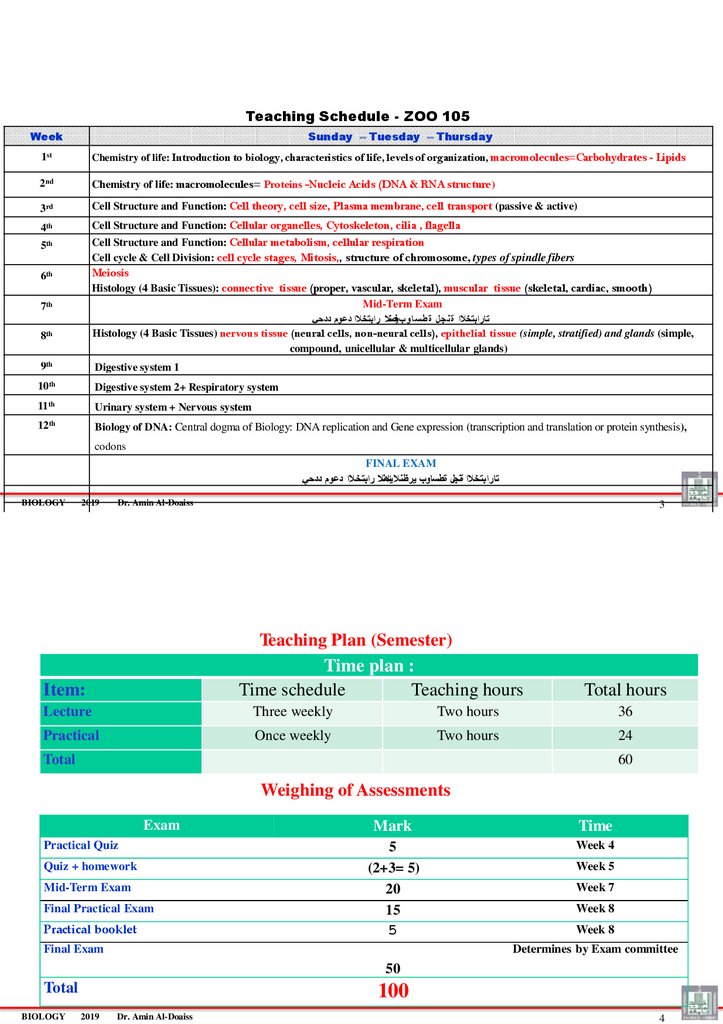
Teaching Schedule - ZOO 105Week
Sunday – Tuesday – Thursday
1st
Chemistry of life: Introduction to biology, characteristics of life, levels of organization, macromolecules=Carbohydrates - Lipids
2nd
Chemistry of life: macromolecules= Proteins -Nucleic Acids (DNA & RNA structure)
3rd
Cell Structure and Function: Cell theory, cell size, Plasma membrane, cell transport (passive & active)
4th
Cell Structure and Function: Cellular organelles, Cytoskeleton, cilia , flagella
5th
Cell Structure and Function: Cellular metabolism, cellular respiration
Cell cycle & Cell Division: cell cycle stages, Mitosis,, structure of chromosome, types of spindle fibers
6th
Meiosis
7th
Histology (4 Basic Tissues): connective tissue (proper, vascular, skeletal), muscular tissue (skeletal, cardiac, smooth)
Mid-Term Exam
تارابتخالا ة نجل ة طس اوبيفصنال رابتخالا دعوم ددحي
8th
Histology (4 Basic Tissues) nervous tissue (neural cells, non-neural cells), epithelial tissue (simple, stratified) and glands (simple,
compound, unicellular & multicellular glands)
9th
Digestive system 1
10th
Digestive system 2+ Respiratory system
11th
Urinary system + Nervous system
12th
Biology of DNA: Central dogma of Biology: DNA replication and Gene expression (transcription and translation or protein synthesis),
codons
FINAL EXAM
تارابتخالا ةنجل ةطساوب يرظنال يئاهنال رابتخالا دعوم ددحي
BIOLOGY
2019
Dr. Amin Al-Doaiss
3
Teaching Plan (Semester)
Time plan :
Time schedule
Teaching hours
Item:
Total hours
Lecture
Three weekly
Two hours
36
Practical
Once weekly
Two hours
24
Total
60
Weighing of Assessments
Exam
Practical Quiz
Quiz + homework
Mid-Term Exam
Final Practical Exam
Practical booklet
Mark
Time
5
(2+3= 5)
20
15
5
Week 4
Final Exam
Week 5
Week 7
Week 8
Week 8
Determines by Exam committee
50
Total
BIOLOGY
100
2019
Dr. Amin Al-Doaiss
4
3.
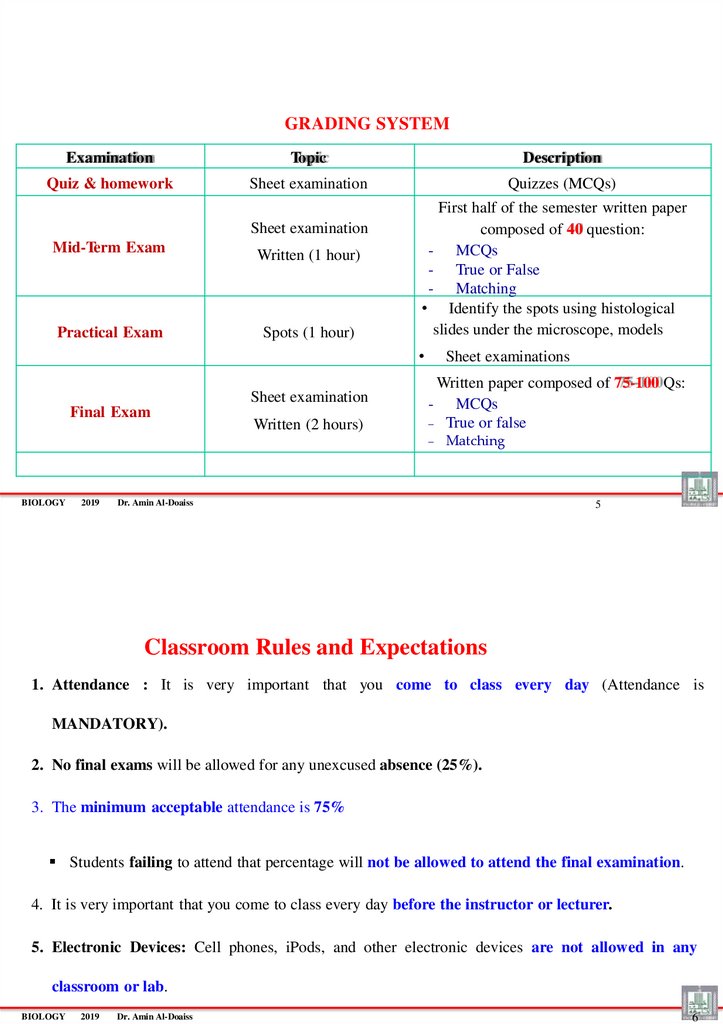
GRADING SYSTEMExamination
Topic
Description
Quiz & homework
Sheet examination
Quizzes (MCQs)
Sheet examination
Mid-Term Exam
Practical Exam
Written (1 hour)
Spots (1 hour)
First half of the semester written paper
composed of 40 question:
- MCQs
- True or False
- Matching
• Identify the spots using histological
slides under the microscope, models
Sheet examination
Final Exam
Written (2 hours)
Sheet examinations
Written paper composed of 75-100 Qs:
- MCQs
True or false
BIOLOGY
2019
Matching
Dr. Amin Al-Doaiss
5
Classroom Rules and Expectations
1. Attendance : It is very important that you come to class every day (Attendance is
MANDATORY).
2. No final exams will be allowed for any unexcused absence (25%).
3. The minimum acceptable attendance is 75%
Students failing to attend that percentage will not be allowed to attend the final examination.
4. It is very important that you come to class every day before the instructor or lecturer.
5. Electronic Devices: Cell phones, iPods, and other electronic devices are not allowed in any
classroom or lab.
BIOLOGY
2019
Dr. Amin Al-Doaiss
6
4.
Introduction to biology•Biology (the study of life)
•The science of biology is the study of living
organisms and their environments.
•living
organisms
have
levels
of
organization: atoms, molecules, cells, tissues,
organs, organ systems, organisms, species,
populations, community, ecosystem, and biosphere.
BIOLOGY
2019
Dr. Amin Al-Doaiss
7
• Biologists classify all life as belonging to one of three domains:
• Domain Bacteria Domain Archaea,
• –Bacteria and Archaea contain prokaryotes = single-celled organisms that lack a nucleus.
–Bacteria and Archaea, belong to kingdom of Monera تايئادبال ةكلمم
•Domain, Eukarya,
– All eukaryotic cells have a true nucleus.
– Some of these organisms are single-celled (unicellular); others are multicellular.
– Eukarya is divided into one of four kingdoms
• Plants (Plantae), fungi (Fungi), animals (Animalia), and protists (Protista) تايعئالطال ةكلمم
• Animalia classified into:
•Invertebrates: Most organisms in kingdom of Animalia are, such as earthworms, insects, mollusks.
•Vertebrates are animals that have a nerve cord protected by a vertebral column, such as
mammals, Fish, reptiles, amphibians, and birds
•Humans are multicelled Eukaryotes, vertebrate, mammalian,
BIOLOGY
2019
Dr. Amin Al-Doaiss
8
5. Chemistry of Life
Dr. AMIN ABDULLAH AL-DOAISSBIOLOGY
2019
Dr. Amin Al-Doaiss
9
From atoms, molecules
to
Human body
BIOLOGY
2019
Dr. Amin Al-Doaiss
10
6.
• Matter is anything that takes up space and has mass.– Matter can exist as a solid, gas, liquid, or plasma (hot ionized gas).
• An element is one of the basic building blocks of matter
– Each element has a name and a symbol (see the Periodic Table)
• An atom is the smallest unit of an element that still retains the chemical and
physical properties of the element.
– The three subatomic particles are: positively charged protons, uncharged neutrons, and negatively charged electrons.
– Protons and neutrons are located within the nucleus of an atom, and electrons move about the
nucleus.
– All atoms of an element have the same number of protons housed in the nucleus. This is called the
atomic number
– The mass number of an atom is the sum of the protons and neutrons in the nucleus.
– Isotopes are the same type of atom have the same number of protons but different numbers of
neutrons.
BIOLOGY
2019
11
Dr. Amin Al-Doaiss
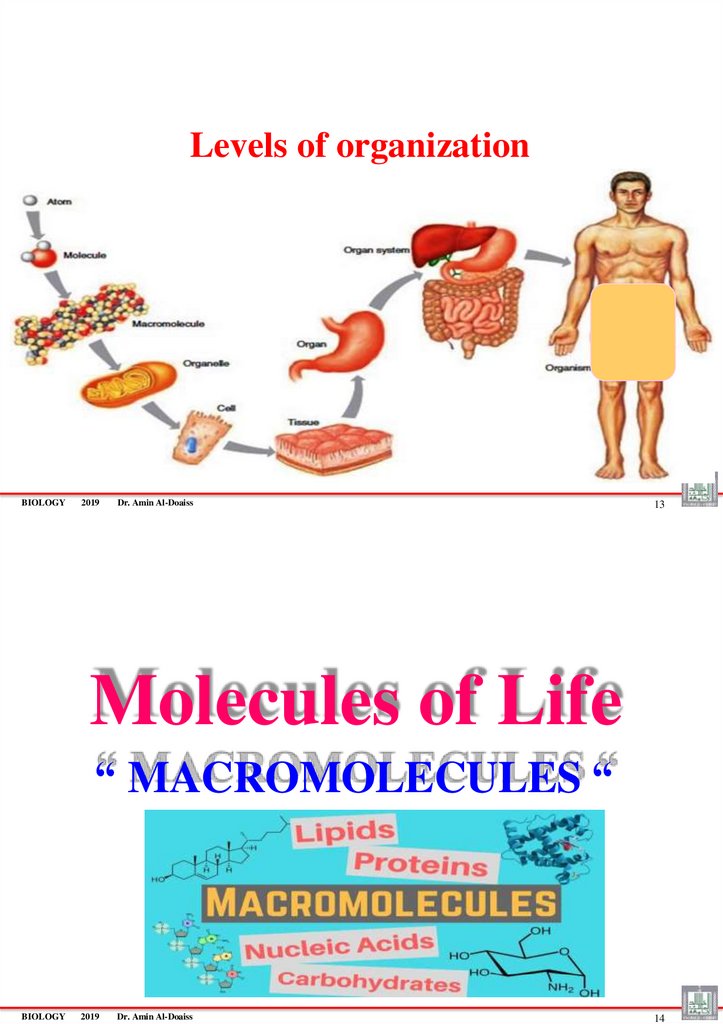
LEVELS OF ORGANIZATION
Hierarchy of structural organization
Atoms & molecules
Macromolecules
Organelles
Cells
Tissues
Organs
Organ Systems
Organism (Human Body)
BIOLOGY
2019
Dr. Amin Al-Doaiss
12
7.
Levels of organizationBIOLOGY
2019
Dr. Amin Al-Doaiss
13
Molecules of Life
“ MACROMOLECULES “
BIOLOGY
2019
Dr. Amin Al-Doaiss
14
8.
MacromoleculesMOLECULES
There are two types of molecules :
1.Inorganic molecules:
• e.g., salts (e.g., NaCl) and water which play important roles
in living things.
2.Organic molecules:
• These always contain carbon (C) and hydrogen (H).
• The molecules of life are organic molecules.
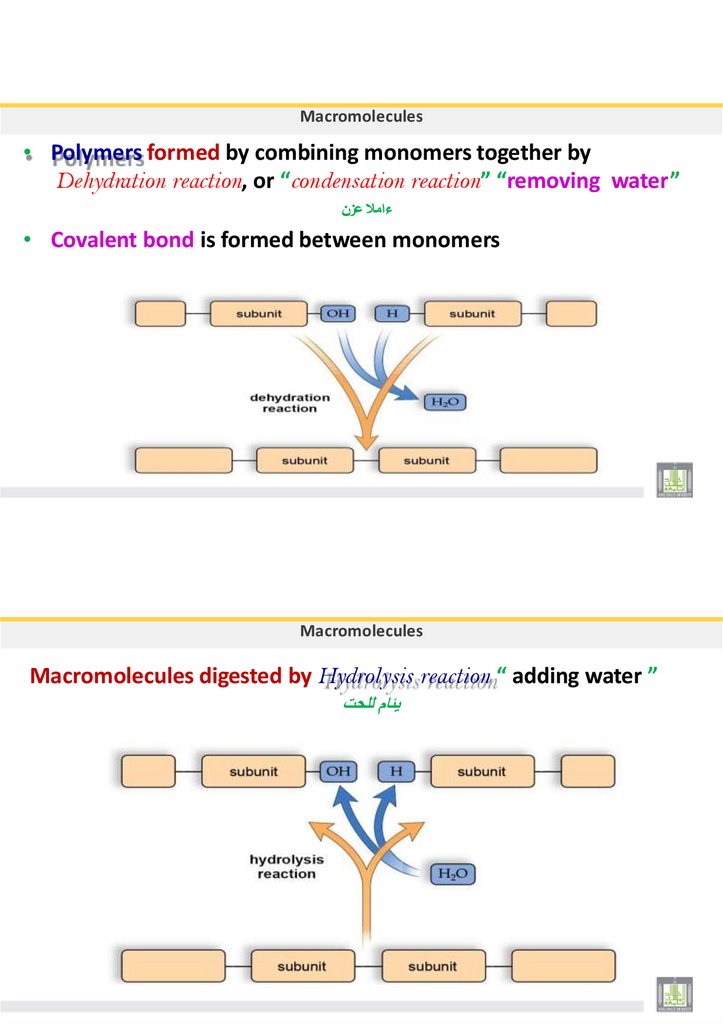
Macromolecules or Polymers
• The large/long organic molecules are called Macromolecules or POLYMERS
Polymer: is a very large/long molecule consisting of many subunits called
MONOMERS bounded by covalent bonds
To synthesis macromolecules, the cell uses a dehydration reaction
To break down and digest the macromolecules, the cell uses a hydrolysis reaction
BIOLOGY
2019
Dr. Amin Al-Doaiss
9.
Macromolecules• Polymers formed by combining monomers together by
Dehydration reaction, or “condensation reaction” “removing water”
ءامال عزن
• Covalent bond is formed between monomers
Macromolecules
Macromolecules digested by Hydrolysis reaction “ adding water ”
يئام للحت
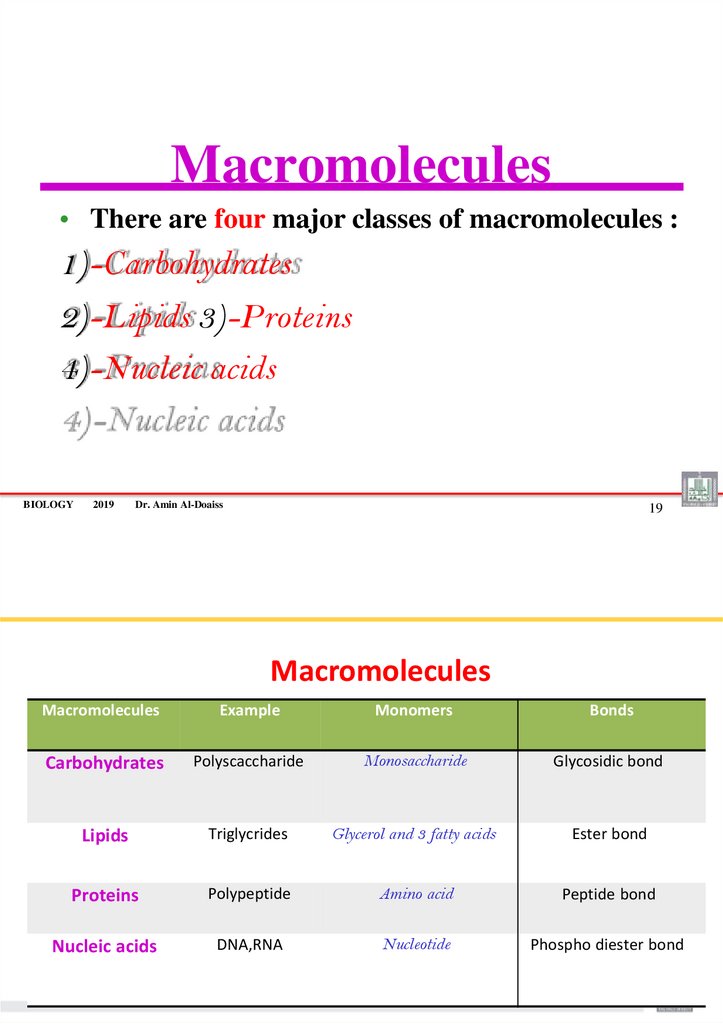
10. Macromolecules
• There are four major classes of macromolecules :1)-Carbohydrates
2)-Lipids 3)-Proteins
4)-Nucleic acids
BIOLOGY
2019
Dr. Amin Al-Doaiss
19
Macromolecules
Macromolecules
Example
Monomers
Bonds
Carbohydrates
Polyscaccharide
Monosaccharide
Glycosidic bond
Lipids
Triglycrides
Glycerol and 3 fatty acids
Ester bond
Proteins
Polypeptide
Amino acid
Peptide bond
Nucleic acids
DNA,RNA
Nucleotide
Phospho diester bond
11.
MacromoleculesBuilding blocks of the cell
Monosaccharaides
Larger units of The cell
POLYSACCHARIDES
2019
Glycosidic bond
FATTY ACIDS
FATS/LIPIDS
Ester bond
AMINO ACIDS
PROTEINS
Peptide bond
NUCLEOTIDES
NUCLEIC ACIDS
Phospho diester
bond
CARBOHYDRATES
BIOLOGY
Chemical Bond
Dr. Amin Al-Doaiss
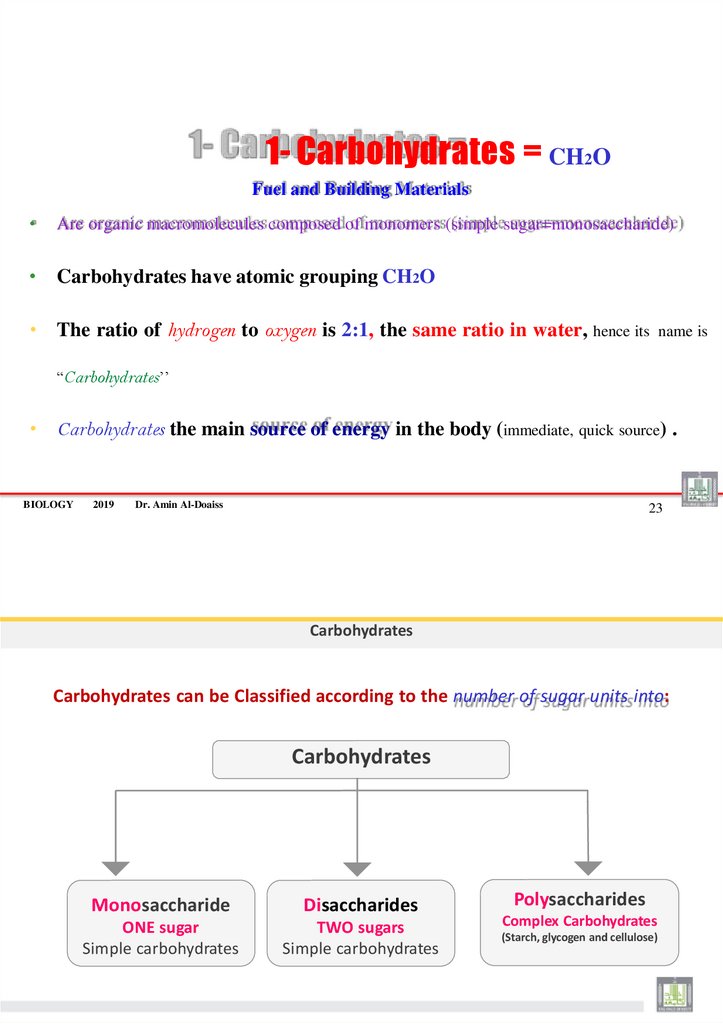
12. 1- Carbohydrates = CH2O
Fuel and Building MaterialsAre organic macromolecules composed of monomers (simple sugar=monosaccharide)
• Carbohydrates have atomic grouping CH2O
• The ratio of hydrogen to oxygen is 2:1, the same ratio in water, hence its
name is
“Carbohydrates’’
• Carbohydrates the main source of energy in the body (immediate, quick source) .
BIOLOGY
2019
Dr. Amin Al-Doaiss
23
Carbohydrates
Carbohydrates can be Classified according to the number of sugar units into:
Carbohydrates
Monosaccharide
Disaccharides
ONE sugar
Simple carbohydrates
TWO sugars
Simple carbohydrates
Polysaccharides
Complex Carbohydrates
(Starch, glycogen and cellulose)
13.
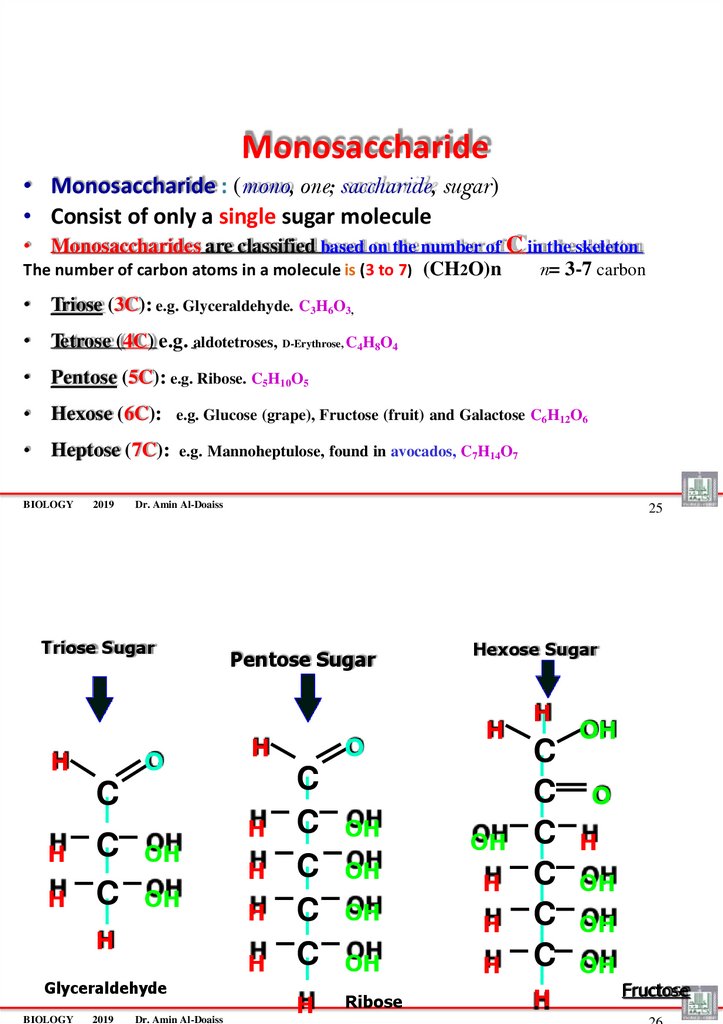
Monosaccharide• Monosaccharide : (mono, one; saccharide, sugar)
• Consist of only a single sugar molecule
• Monosaccharides are classified based on the number of C in the skeleton
The number of carbon atoms in a molecule is (3 to 7) (CH2O)n
n= 3-7 carbon
• Triose (3C): e.g. Glyceraldehyde. C3H6O3,
• Tetrose (4C) e.g. aldotetroses, D-Erythrose, C4H8O4
• Pentose (5C): e.g. Ribose. C5H10O5
• Hexose (6C):
e.g. Glucose (grape), Fructose (fruit) and Galactose C6H12O6
• Heptose (7C):
e.g. Mannoheptulose, found in avocados, C7H14O7
BIOLOGY
2019
Dr. Amin Al-Doaiss
Triose Sugar
H
O
25
Pentose Sugar
H
C
H
H
C
C
OH
OH
H
2019
H
H
H
Glyceraldehyde
BIOLOGY
H
Dr. Amin Al-Doaiss
O
C
C
C
C
C
H
OH
OH
Hexose Sugar
H
OH
H
OH
H
OH
H
Ribose
H
C
C
C
C
C
C
H
OH
O
H
OH
OH
OH
Fructose
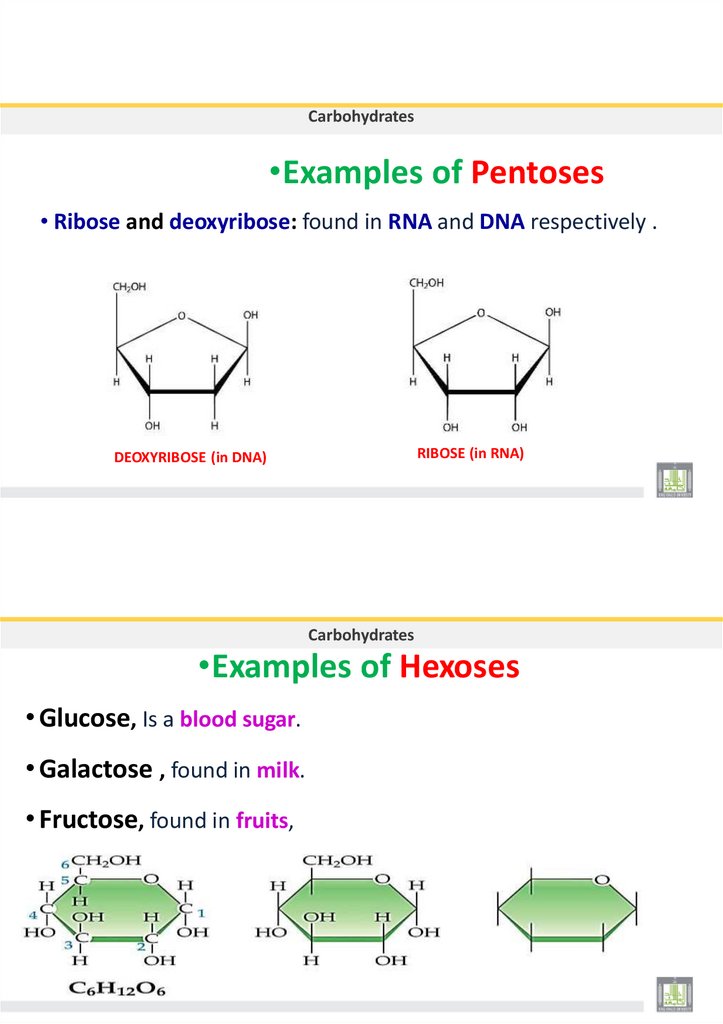
14. •Examples of Pentoses
Carbohydrates•Examples of Pentoses
• Ribose and deoxyribose: found in RNA and DNA respectively .
RIBOSE (in RNA)
DEOXYRIBOSE (in DNA)
Carbohydrates
•Examples of Hexoses
• Glucose, Is a blood sugar.
• Galactose , found in milk.
• Fructose, found in fruits,
15.
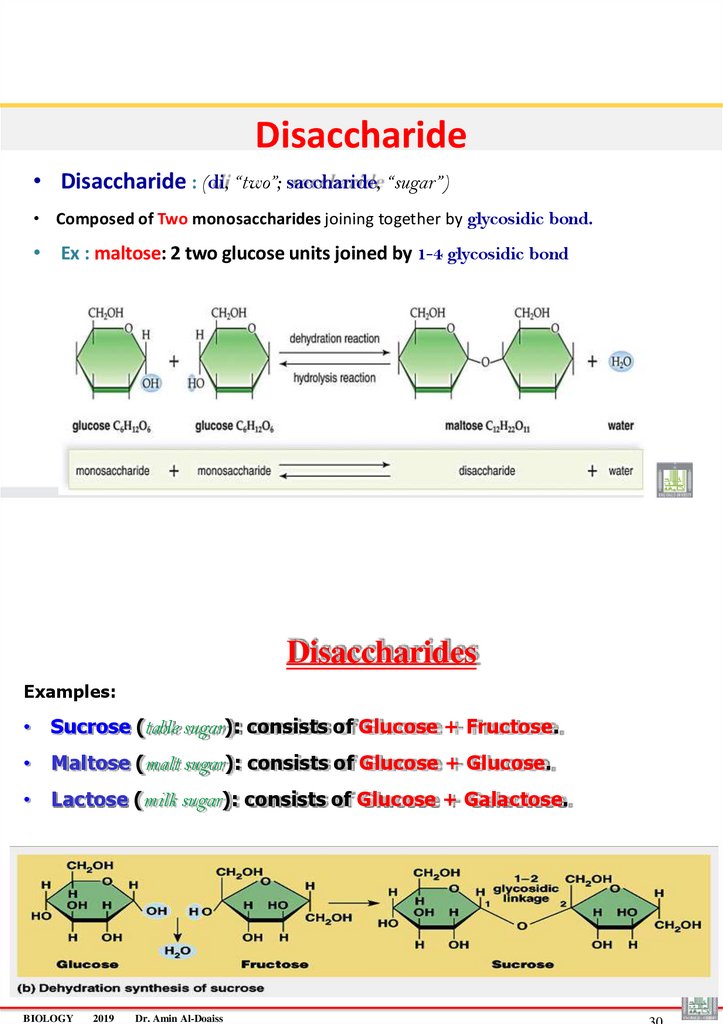
Disaccharide• Disaccharide : (di, “two”; saccharide, “sugar”)
• Composed of Two monosaccharides joining together by glycosidic bond.
• Ex : maltose: 2 two glucose units joined by 1-4 glycosidic bond
Disaccharides
Examples:
• Sucrose (table sugar): consists of Glucose + Fructose.
• Maltose (malt sugar): consists of Glucose + Glucose.
• Lactose (milk sugar): consists of Glucose + Galactose.
BIOLOGY
2019
Dr. Amin Al-Doaiss
16.
DisaccharidesDisaccharide
Monomer
Common name
Maltose
Glucose + Glucose
Lactose
Glucose + Galactose
sugar
Sucrose
Glucose + Fructose
sugarcane
Malt sugar Milk
.
Example:
• When glucose and fructose join, the disaccharide sucrose forms
• Sucrose, derived from sugarcane and sugar beets, is known as table sugar.
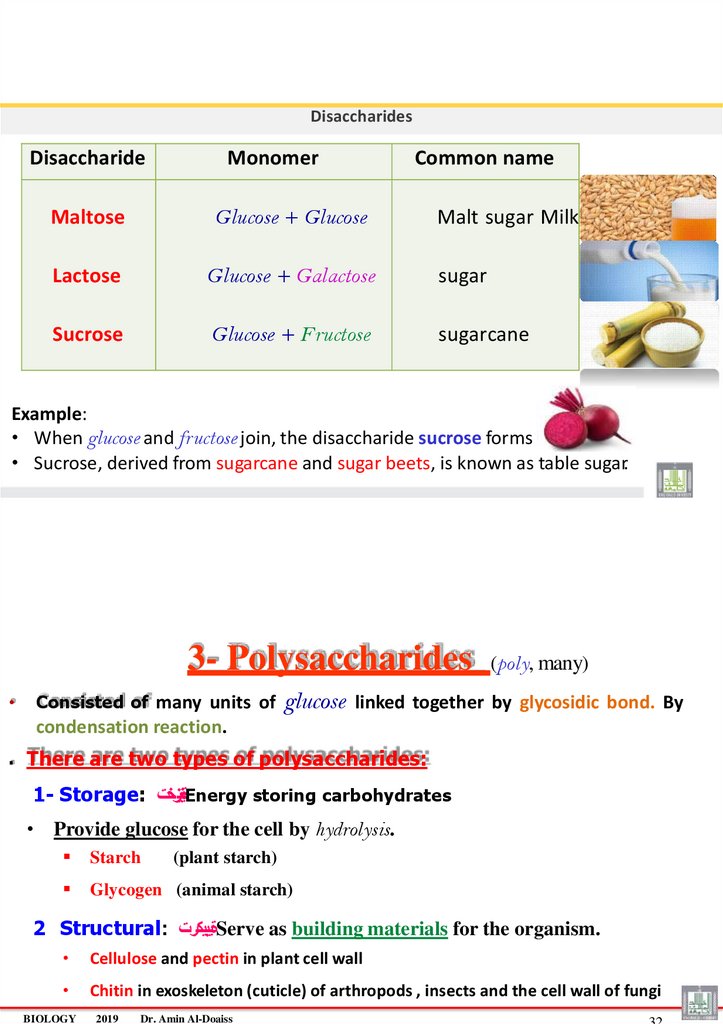
3- Polysaccharides
Consisted of many units of
(poly, many)
glucose linked together by glycosidic bond. By
condensation reaction.
. There are two types of polysaccharides:
1- Storage: ةينيزختEnergy storing carbohydrates
• Provide glucose for the cell by hydrolysis.
Starch
Glycogen (animal starch)
(plant starch)
2 Structural: ةيبيكرتServe as building materials for the organism.
Cellulose and pectin in plant cell wall
Chitin in exoskeleton (cuticle) of arthropods , insects and the cell wall of fungi
BIOLOGY
2019
Dr. Amin Al-Doaiss
17.
CarbohydratesSTARCH :
•It is a Long chain of glucose (up to 4,000 units) linked together by glycosidic
bonds.
•Found in many grains بوبحand tubers تانرد.
•Starch is the most common carbohydrate in the human diet.
•Animals can be digest starch into glucose subunits (by amylase enzyme) to use as an
energy source
Carbohydrates
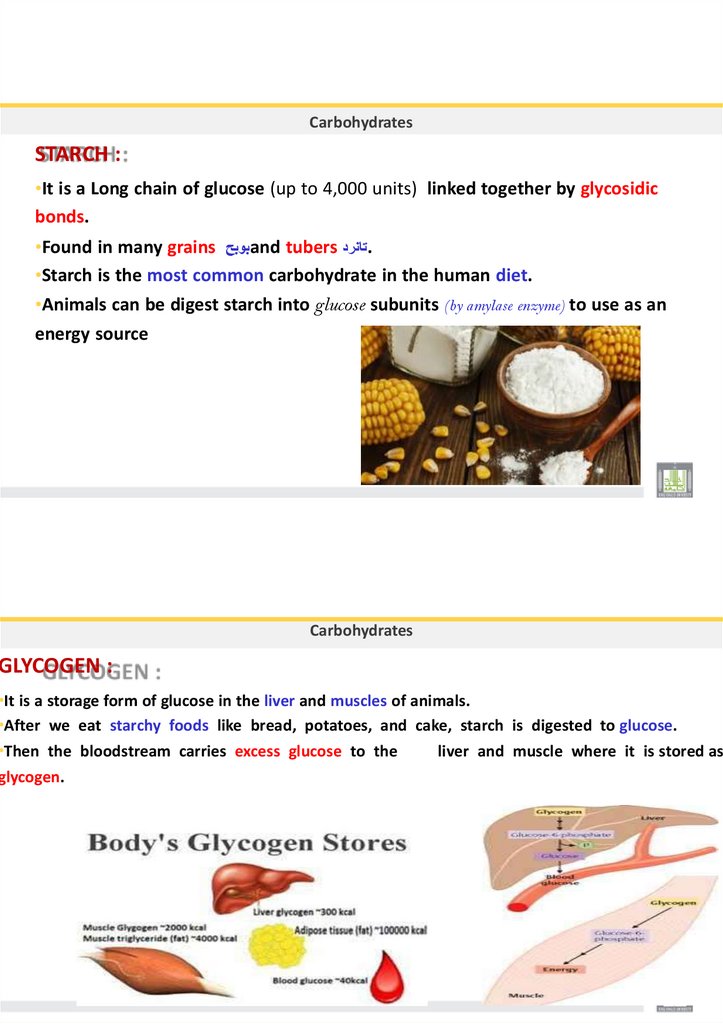
GLYCOGEN :
•It is a storage form of glucose in the liver and muscles of animals.
•After we eat starchy foods like bread, potatoes, and cake, starch is digested to glucose.
•Then the bloodstream carries excess glucose to the
liver and muscle where it is stored as
glycogen.
18.
CarbohydratesCELLULOSE :
It is a structural form of polysaccharide, supports the plant cell wall
Cellulose is the most common organic compound on Earth.
Cellulose passes through our digestive tract as fibers.
Humans cannot digest cellulose because they lack the enzyme cellulase.
but it is important in the healthy diet because it is a source of fibres.
سيعدال اللهدبع نيمأ.د
Dr. AMIN ABDULLAH AL-DOAISS
1
19.
LipidsBIOLOGY
2019
Dr. Amin Al-Doaiss
2
Lipids
• Fats or lipids are long chains of hydrocarbons (16 -18 C per molecule)
• Each molecule ending in - COOH group
• They contain little oxygen and mostly of carbon and hydrogen atoms.
• Lipids contain more energy than other biological molecules (energy source).
• There are Different types:
e.g., 1
2
3
4
5
6
Triglycerides
Oils
Waxes
Phospholipids
Steroid hormones
Fat-soluble vitamins
Phospholipids are type of lipids that form the major components of cell membranes
Steroids are type of lipids that contains cholesterol and form the sex hormones.
BIOLOG
3Y
2019
Dr. Amin Al-Doaiss
20.
LipidsFunctions of lipids :
1.Long term energy source
2.Prevent heat loss (Protection-insulation
3.Prevent water loss
4.Support
and
cushioning
cushioning
for bones
5.Form sex hormones (steroids)
6.Major co
ranes
(p of cell memb
mponent
BIOLOGY
2019
Dr. Amin
for
organs and
hospholipids).
4
Al-Doaiss
Triglycerides
)
ةيثالثال نوهدال
The most common lipids are called the triglycerides.
There are Two types:
n)
1 Fats :(Solid at room temperature, animal origi
e.g., Butter
n)
2 Oils : (Liquid at room temperature, plant origi
e.g., vegetable oils, olive oil, corn oil, sunflower oil, sesame oil
BIOLOGY
2019
Dr. Amin Al-Doaiss
21.
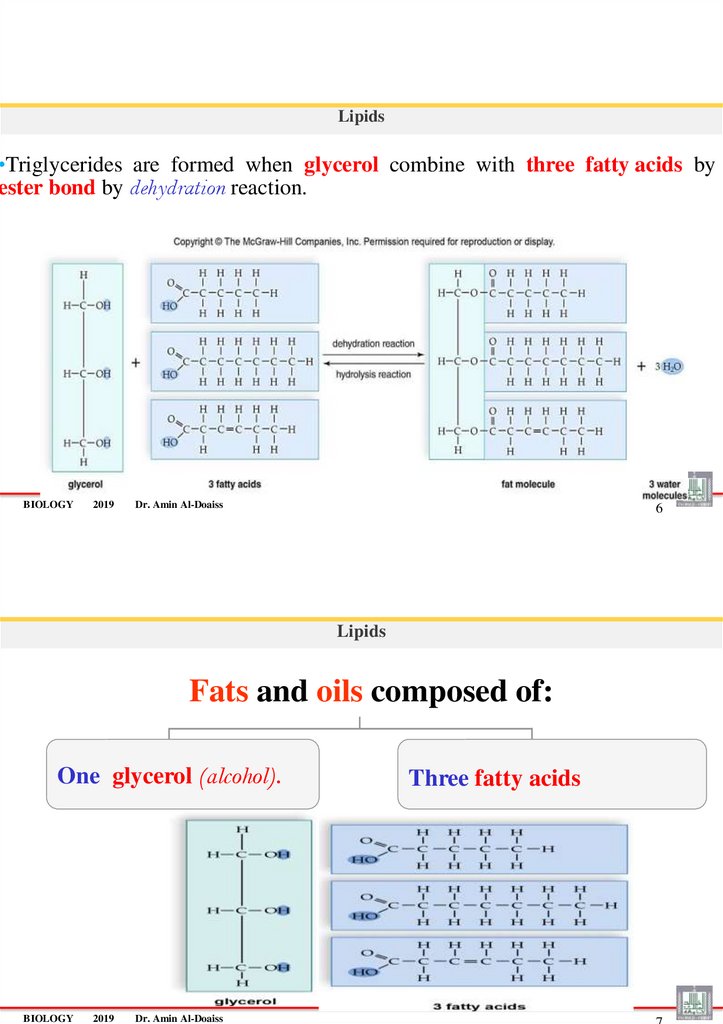
Lipids•Triglycerides are formed when glycerol combine with three fatty acids by
ester bond by dehydration reaction.
BIOLOGY
2019
Dr. Amin Al-Doaiss
6
Lipids
Fats and oils composed of:
One glycerol (alcohol).
BIOLOGY
2019
Dr. Amin Al-Doaiss
Three fatty acids
22.
GlycerolO
H
H
C
C
H
OH
Ester link
C
OH
H
H
O
H
C
OH
C
C H
H
O
H
H
C
CDehydration
C
H
BIOLOGY
2019
H
C
H
H
C
H
C
H
H
C
H
H
H
H
C
H
C
H
H
C
H
H
C
H
H
H
C
H
H
Fatty Acid
H
C
H Fatty Acid
H
H
C
H
C
H Fatty Acid
H
Dr. Amin Al-Doaiss
8
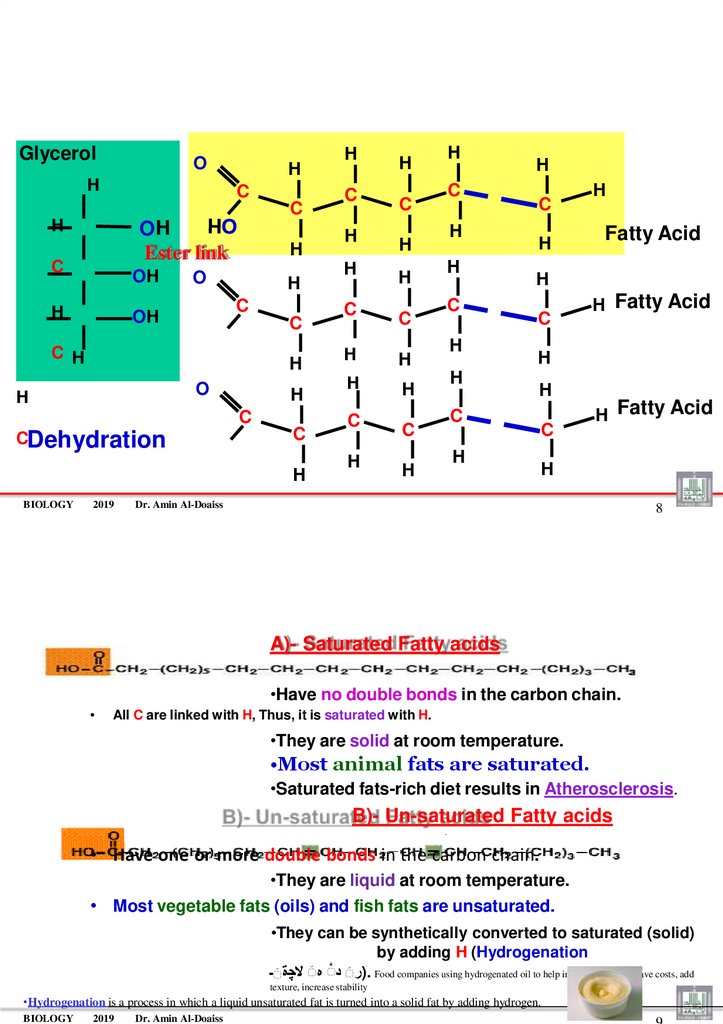
A)- Saturated Fatty acids
•Have no double bonds in the carbon chain.
All C are linked with H, Thus, it is saturated with H.
•They are solid at room temperature.
•Most animal fats are saturated.
•Saturated fats-rich diet results in Atherosclerosis.
B)- Un-saturated Fatty acids
• Have one or more double bonds in the carbon chain.
•They are liquid at room temperature.
• Most vegetable fats (oils) and fish fats are unsaturated.
•They can be synthetically converted to saturated (solid)
by adding H (Hydrogenation
)رَ دَ هَ ال ةَـ. Food companies using hydrogenated oil to help increase shelf life, save costs, add
texture, increase stability
•Hydrogenation is a process in which a liquid unsaturated fat is turned into a solid fat by adding hydrogen.
BIOLOGY
2019
Dr. Amin Al-Doaiss
23.
بلحتسمال و تابلَ حتسمال و بالحتسالاEmulsifiers, Emulsification & Emulsion
•Emulsifiers are substances can cause fats to mix with water.
•Because the
mulsifiers contain both
nonpolar
(lipophilic) tail
and
polar
(hydrophilic) head.
•nonpolar tails surround the oil droplet but the polar heads project toward
the
water.
•Now the lipid droplet transports in water, which means that emulsification
has occurred and the product is called emulsion.
•Fats are emulsified by bile (broken down the large drop into droplets)
Polar end
Nonpolar end
emulsion
+
lipid
10
An emulsion is a mixture of two or more liquids that are normally immiscible
2019
BIOLOG
11Y
Dr. Amin Al-Doaiss
24.
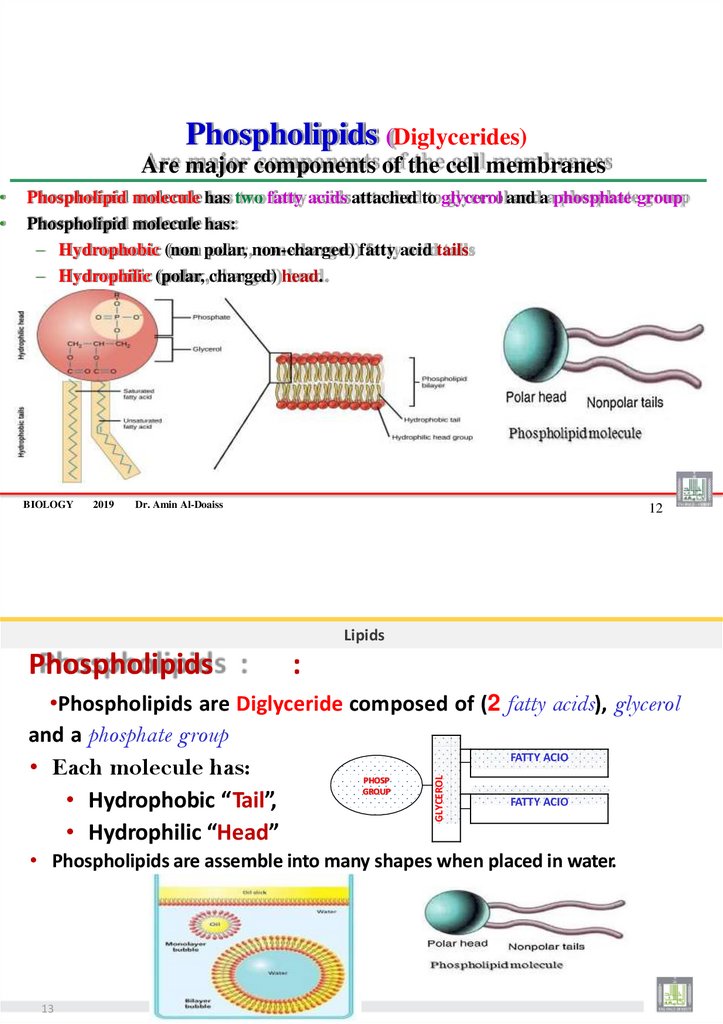
Phospholipids (Diglycerides)Are major components of the cell membranes
Phospholipid molecule has two fatty acids attached to glycerol and a phosphate group
Phospholipid molecule has:
– Hydrophobic (non polar, non-charged) fatty acid tails
– Hydrophilic (polar, charged) head.
BIOLOGY
2019
Dr. Amin Al-Doaiss
12
Lipids
Phospholipids
:
•Phospholipids are Diglyceride composed of (2 fatty acids), glycerol
and a phosphate group
FATTY ACIO
• Each molecule has:
FATTY ACIO
• Hydrophobic “Tail”,
• Hydrophilic “Head”
PHOSP
GROUP
GLYCEROL
• Phospholipids are assemble into many shapes when placed in water.
13
25.
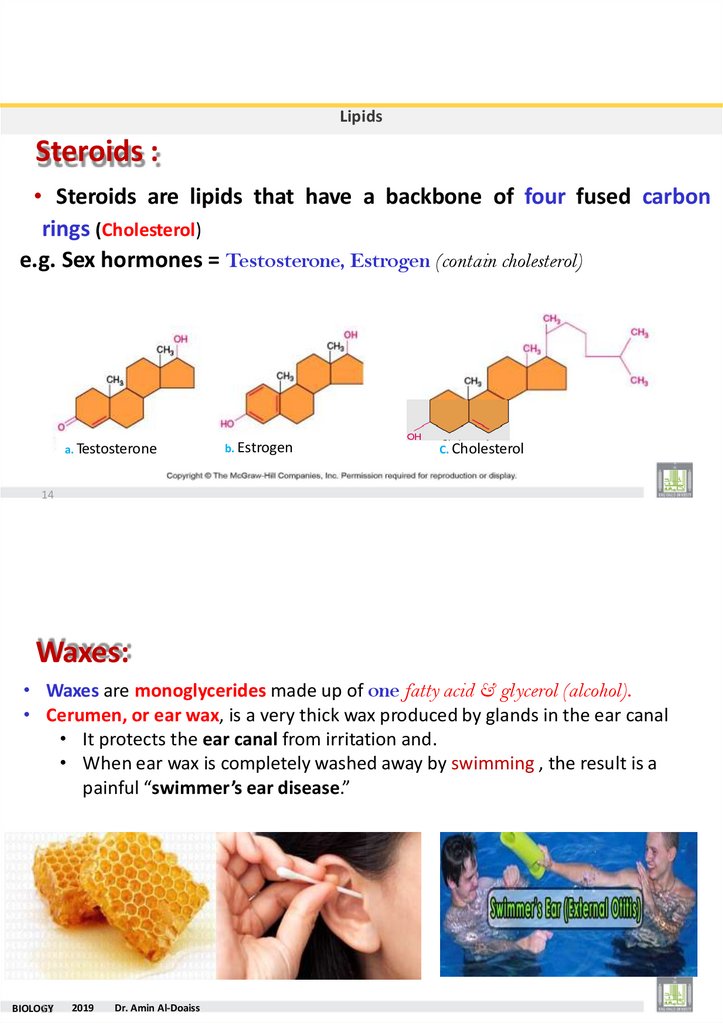
LipidsSteroids :
• Steroids are lipids that have a backbone of four fused carbon
rings (Cholesterol)
e.g. Sex hormones = Testosterone, Estrogen (contain cholesterol)
a. Testosterone
b. Estrogen
C. Cholesterol
14
Waxes:
• Waxes are monoglycerides made up of one fatty acid & glycerol (alcohol).
• Cerumen, or ear wax, is a very thick wax produced by glands in the ear canal
• It protects the ear canal from irritation and.
• When ear wax is completely washed away by swimming , the result is a
painful “swimmer’s ear disease.”
BIOLOG
15Y
2019
Dr. Amin Al-Doaiss
26.
The Omega-3 Fatty AcidsA special class of unsaturated fatty acids, the omega-3 fatty
acids, is an essential for
healthy heart.
•The name omega-3 is derived from the location of the
double bond in the carbon chain.
•Some of the best source of omega-3 fatty acids is fish oil.
BIOLOGY
2019
Dr. Amin Al-Doaiss
16
Proteins
BIOLOG
17Y
2019
Dr. Amin Al-Doaiss
27.
2-Proteins- Protein is a polymer of amino acids (constructed from 20 amino acids) (to form
Polypeptides).
- Amino Acid composed of: a hydrogen atom, an amino group, a carboxyl group, and a
variable R group (or side chain).
H
H
General Formula of
the Amino Acid:
N
C
H
O
C
R
Amino
group
Side chain
OH
Carboxyl
group
- The side chain R links with different compounds
- Differences in R groups produce the 20 different amino acids.
BIOLOGY
2019
Dr. Amin Al-Doaiss
18
Proteins
Functions of proteins :
1.
Storage:
Albumin (egg white)
2.
Transport:
3.
Regulatory: Hormones (intercellualr messengers)
Channel and carrier proteins Hemoglobin
e.g., insulin and growth hormone
4.
Motion :
5.
Support : hair, nails (keratin), skin and ligaments (collagen)
6.
Enzymes:
Cellular chemical reactions (specific)
7.
Defense :
Antibodies.
19
Muscles (Actin and myosin)
28.
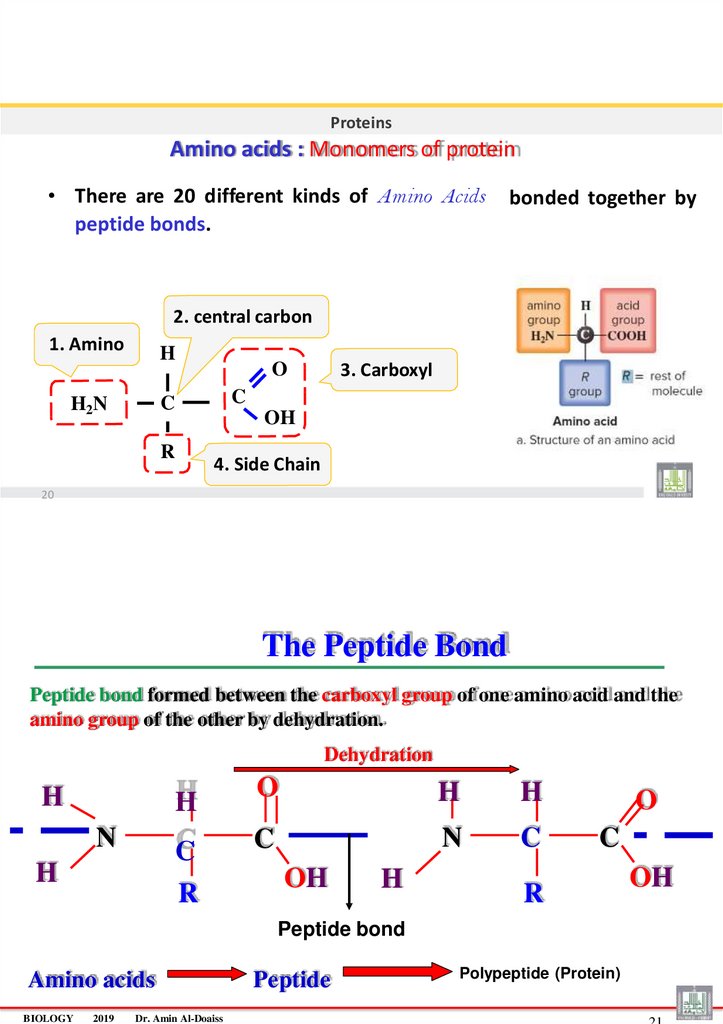
ProteinsAmino acids : Monomers of protein
• There are 20 different kinds of Amino Acids
peptide bonds.
bonded together by
2. central carbon
1. Amino
H
C
C
H2N
3. Carboxyl
O
OH
R
4. Side Chain
20
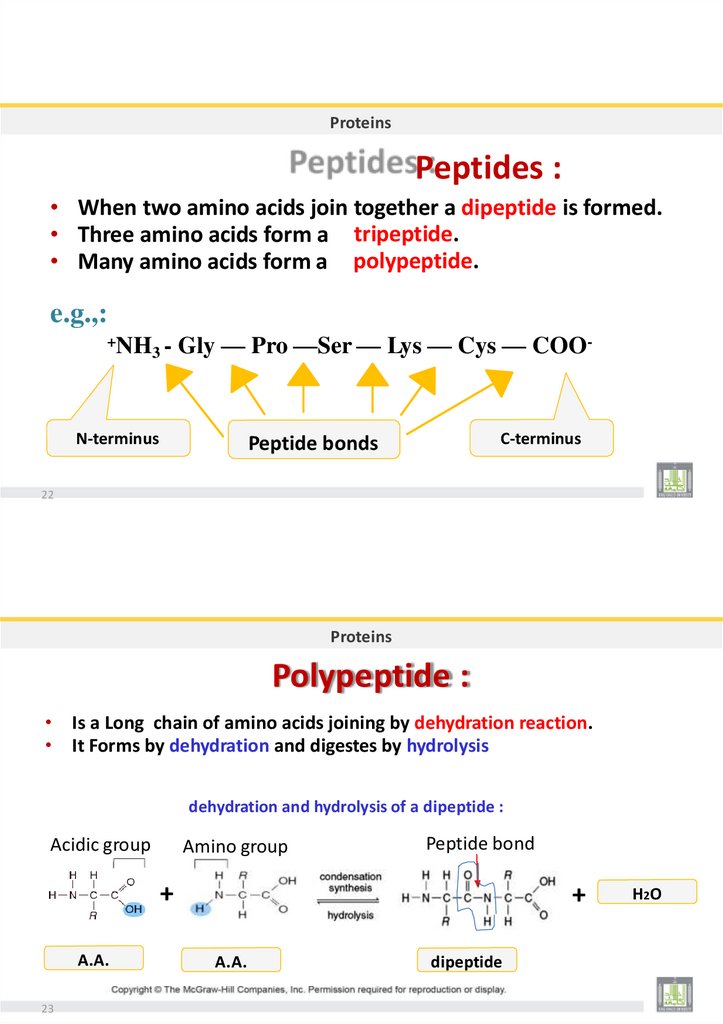
The Peptide Bond
Peptide bond formed between the carboxyl group of one amino acid and the
amino group of the other by dehydration.
Dehydration
H
H
N
C
H
R
O
H
H
C
N
C
OH
H
O
C
R
Peptide bond
Amino acids
BIOLOGY
2019
Dr. Amin Al-Doaiss
Peptide
Polypeptide (Protein)
OH
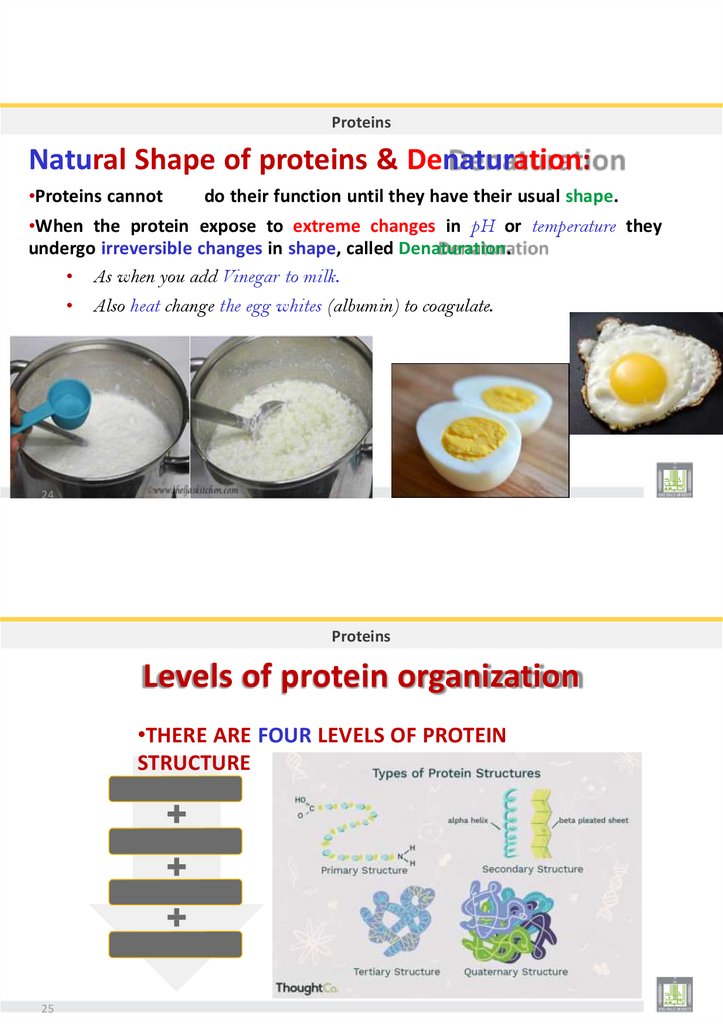
29. Peptides :
ProteinsPeptides :
• When two amino acids join together a dipeptide is formed.
• Three amino acids form a tripeptide.
• Many amino acids form a polypeptide.
e.g.,:
+NH
3-
Gly — Pro —Ser — Lys — Cys — COO-
N-terminus
C-terminus
Peptide bonds
22
Proteins
Polypeptide :
• Is a Long chain of amino acids joining by dehydration reaction.
• It Forms by dehydration and digestes by hydrolysis
dehydration and hydrolysis of a dipeptide :
Acidic group
Amino group
Peptide bond
+
A.A.
23
+
A.A.
dipeptide
H2O
30.
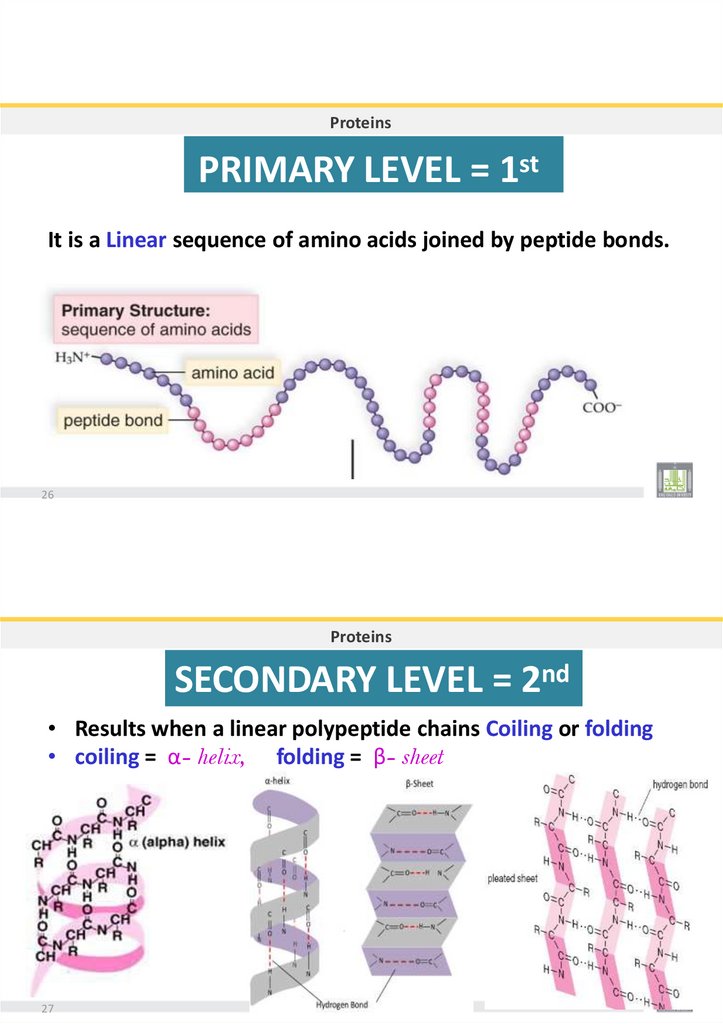
ProteinsNatural Shape of proteins & Denaturation:
•Proteins cannot
do their function until they have their usual shape.
•When the protein expose to extreme changes in pH or temperature they
undergo irreversible changes in shape, called Denaturation.
• As when you add Vinegar to milk.
Also heat change the egg whites (albumin) to coagulate.
24
Proteins
Levels of protein organization
•THERE ARE FOUR LEVELS OF PROTEIN
STRUCTURE
PRIMARY
SECONDARY TERTIAR QUATERNARY
25
31.
ProteinsPRIMARY LEVEL = 1st
It is a Linear sequence of amino acids joined by peptide bonds.
26
Proteins
SECONDARY LEVEL = 2nd
• Results when a linear polypeptide chains Coiling or folding
• coiling = α- helix, folding = β- sheet
27
32.
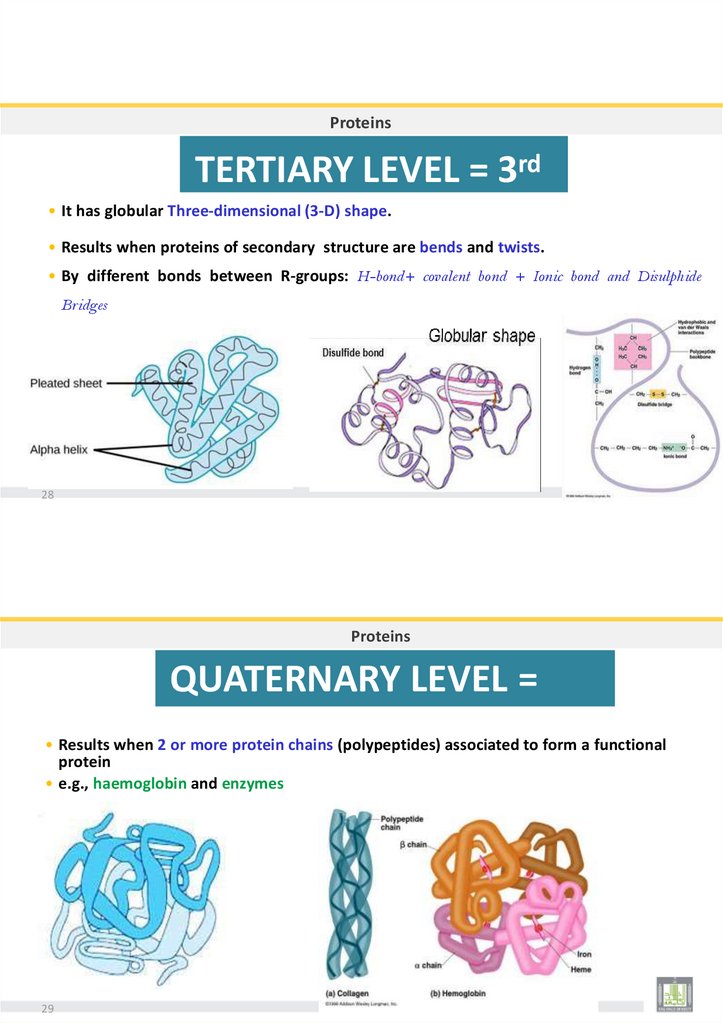
ProteinsTERTIARY LEVEL = 3rd
• It has globular Three-dimensional (3-D) shape.
• Results when proteins of secondary structure are bends and twists.
• By different bonds between R-groups: H-bond+ covalent bond + Ionic bond and Disulphide
Bridges
28
Proteins
QUATERNARY LEVEL =
th
4
• Results when 2 or more protein chains (polypeptides) associated to form a functional
protein
• e.g., haemoglobin and enzymes
29
33.
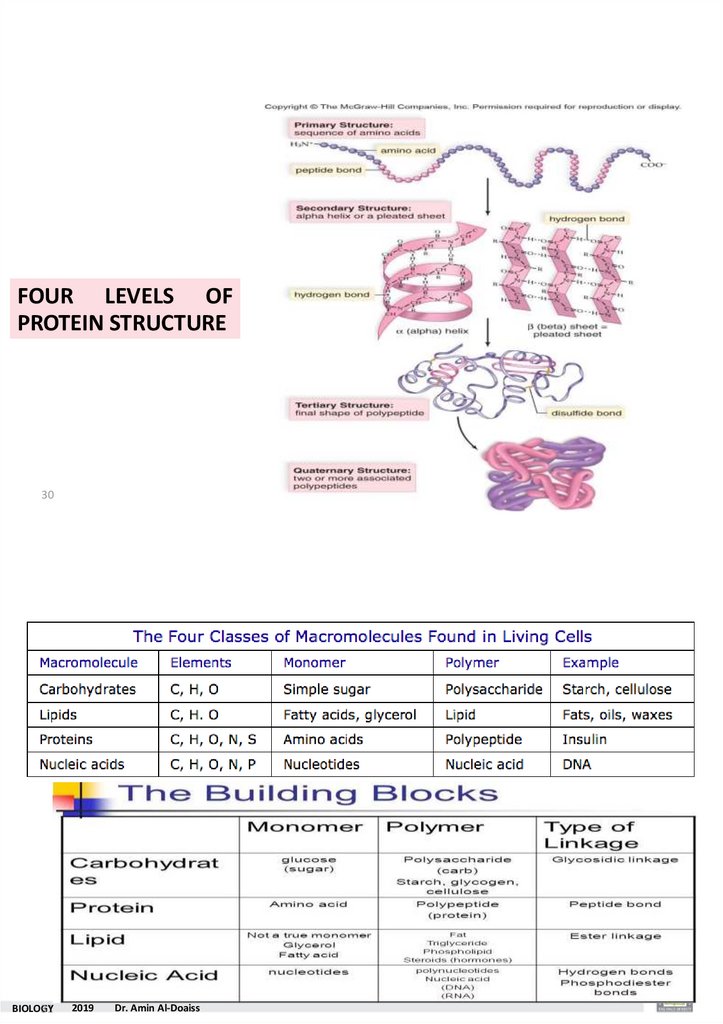
FOUR LEVELS OFPROTEIN STRUCTURE
30
BIOLOG
31Y
2019
Dr. Amin Al-Doaiss
34.
n Al-DoaissBIOLO
3G
2Y
2019
Dr. Ami
Dr. AMIN ABDULLAH AL-DOAISS
35.
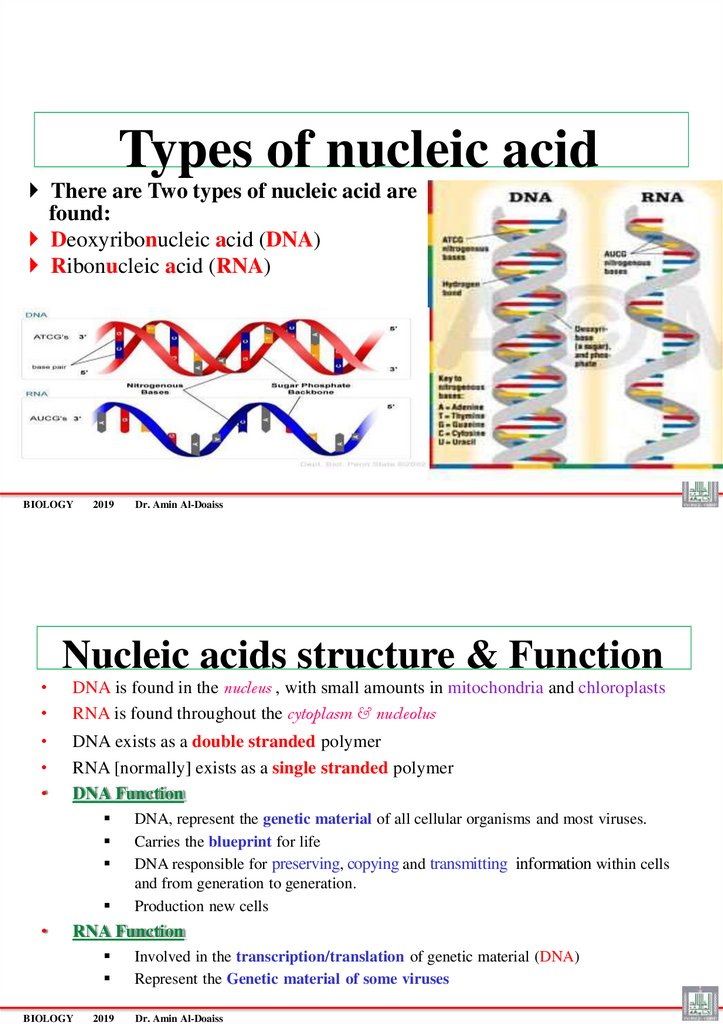
Types of nucleic acidThere are Two types of nucleic acid are
found:
Deoxyribonucleic acid (DNA)
Ribonucleic acid (RNA)
BIOLOGY
2019
Dr. Amin Al-Doaiss
Nucleic acids structure & Function
DNA is found in the nucleus , with small amounts in mitochondria and chloroplasts
RNA is found throughout the cytoplasm & nucleolus
DNA exists as a double stranded polymer
RNA [normally] exists as a single stranded polymer
DNA Function
DNA, represent the genetic material of all cellular organisms and most viruses.
Carries the blueprint for life
DNA responsible for preserving, copying and transmitting information within cells
and from generation to generation.
Production new cells
RNA Function
BIOLOGY
2019
Involved in the transcription/translation of genetic material (DNA)
Represent the Genetic material of some viruses
Dr. Amin Al-Doaiss
36.
DNA (Deoxyribonucleic Acid)1953- Watson and Crick created a 3-D
model of structure called the double helix
Watson-Crick : Nobel’s prize for double helix structure
37.
Amin Al-DoaissJames Watson
(at King Saud University, Riyadh, Saudi Arabia, 2012)
BIOLOGY
2019
Dr. Amin Al-Doaiss
The Watson-Crick Model of DNA Structure
• DNA has a double helix structure.
• It is consist of two complementary anti-parallel strands
• The sides of the ladder are made up of alternating molecules of
phosphate and sugar (S-P-S-P-S-P…)
3
5
5
3
The rungs of the ladder consist of nitrogenous bases connected by
Hydrogen bonds
3
Sugar phosphate Backbone
5
Sugar phosphate Backbone
3
rungs
5
BIOLOGY
2019
Dr. Amin Al-Doaiss
38.
BIOLOGY2019
Dr. Amin Al-Doaiss
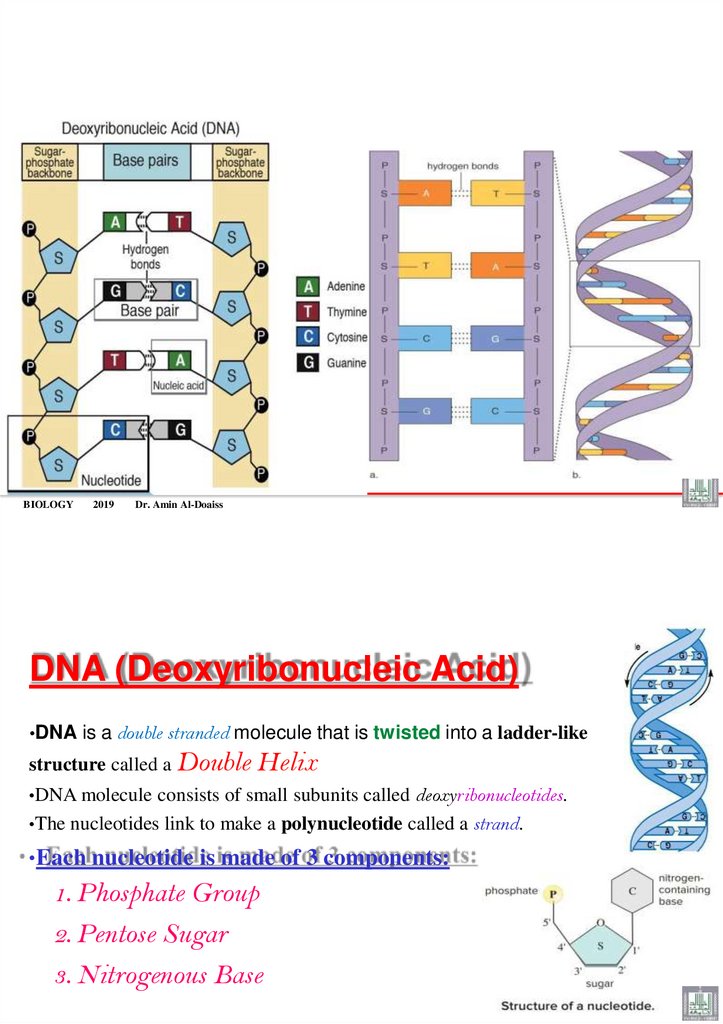
DNA (Deoxyribonucleic Acid)
•DNA is a double stranded molecule that is twisted into a ladder-like
structure called a Double
Helix
•DNA molecule consists of small subunits called deoxyribonucleotides.
•The nucleotides link to make a polynucleotide called a strand.
•Each nucleotide is made of 3 components:
1. Phosphate Group
2. Pentose Sugar
3. Nitrogenous Base
39.
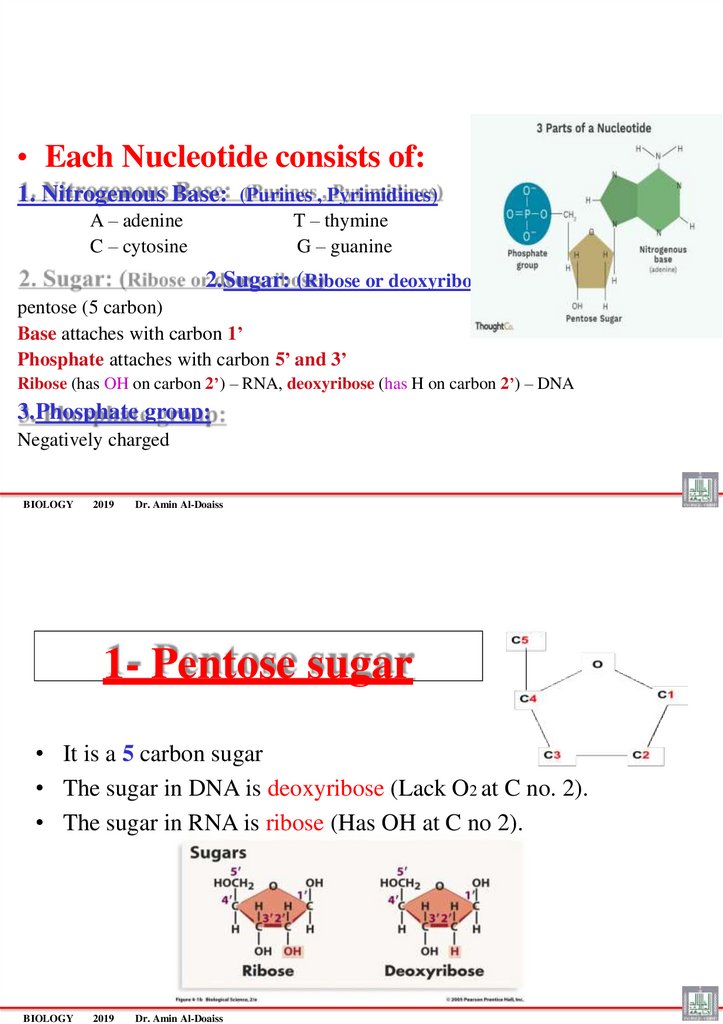
• Each Nucleotide consists of:1. Nitrogenous Base: (Purines , Pyrimidines)
A – adenine
C – cytosine
T – thymine
G – guanine
2.Sugar: (Ribose or deoxyribose)
pentose (5 carbon)
Base attaches with carbon 1’
Phosphate attaches with carbon 5’ and 3’
Ribose (has OH on carbon 2’) – RNA, deoxyribose (has H on carbon 2’) – DNA
3.Phosphate group:
Negatively charged
BIOLOGY
2019
Dr. Amin Al-Doaiss
1- Pentose sugar
• It is a 5 carbon sugar
• The sugar in DNA is deoxyribose (Lack O2 at C no. 2).
• The sugar in RNA is ribose (Has OH at C no 2).
BIOLOGY
2019
Dr. Amin Al-Doaiss
40.
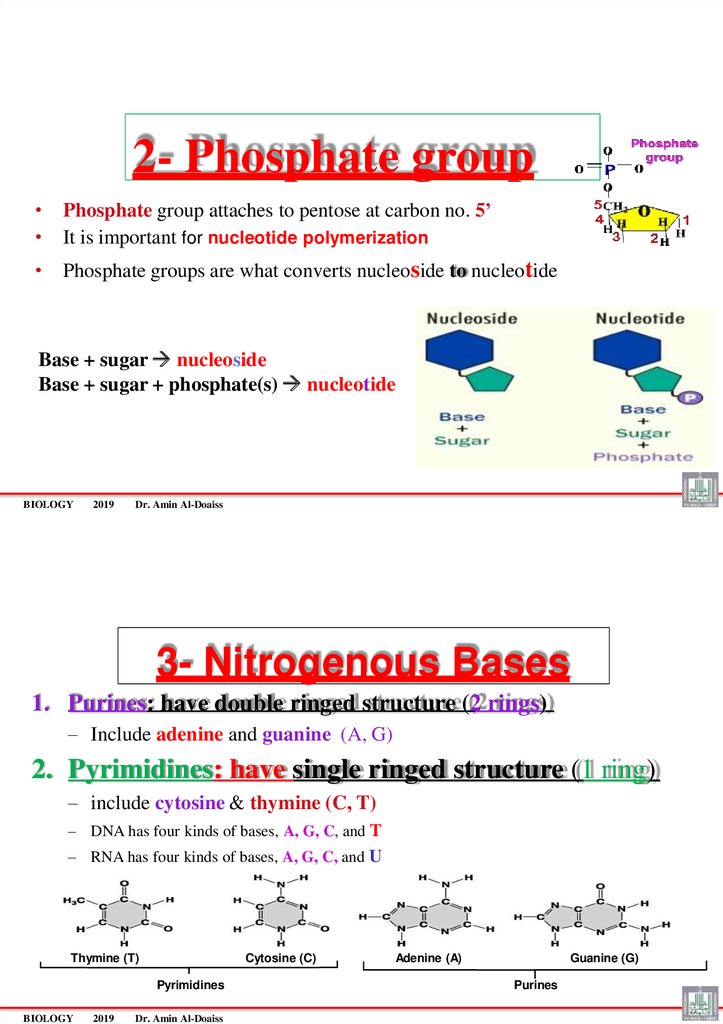
2- Phosphate group• Phosphate group attaches to pentose at carbon no. 5’
• It is important for nucleotide polymerization
• Phosphate groups are what converts nucleoside to nucleotide
Base + sugar nucleoside
Base + sugar + phosphate(s) nucleotide
BIOLOGY
2019
Dr. Amin Al-Doaiss
3- Nitrogenous Bases
1. Purines: have double ringed structure (2 rings)
– Include adenine and guanine (A, G)
2. Pyrimidines: have single ringed structure (1 ring)
– include cytosine & thymine (C, T)
– DNA has four kinds of bases, A, G, C, and T
– RNA has four kinds of bases, A, G, C, and U
Thymine (T)
Cytosine (C)
Pyrimidines
BIOLOGY
2019
Dr. Amin Al-Doaiss
Adenine (A)
Guanine (G)
Purines
41.
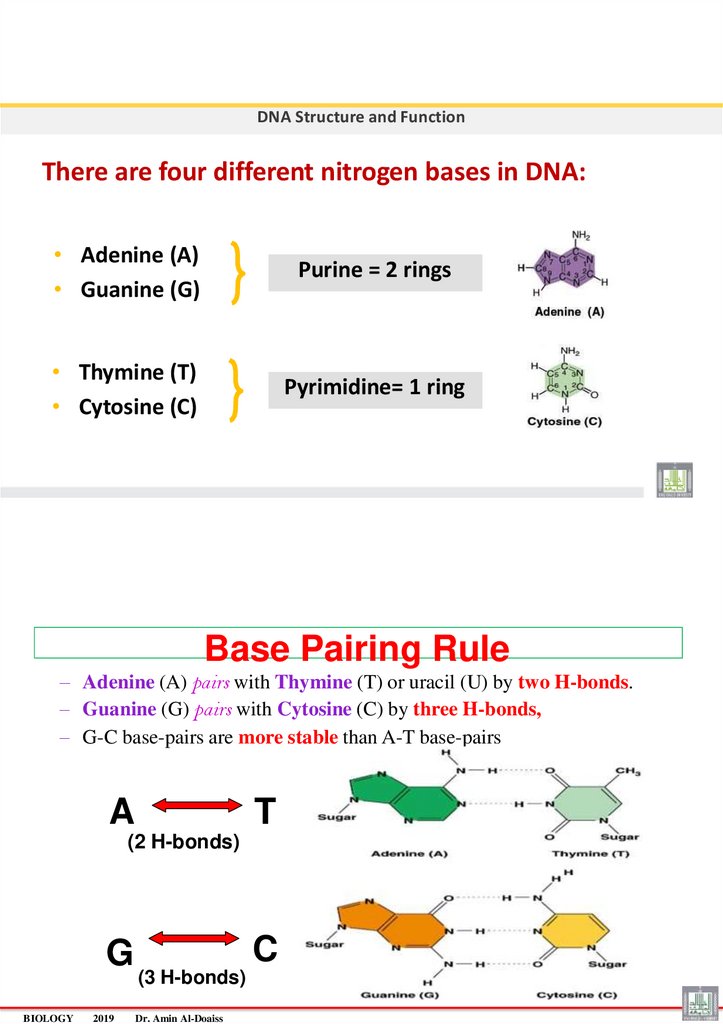
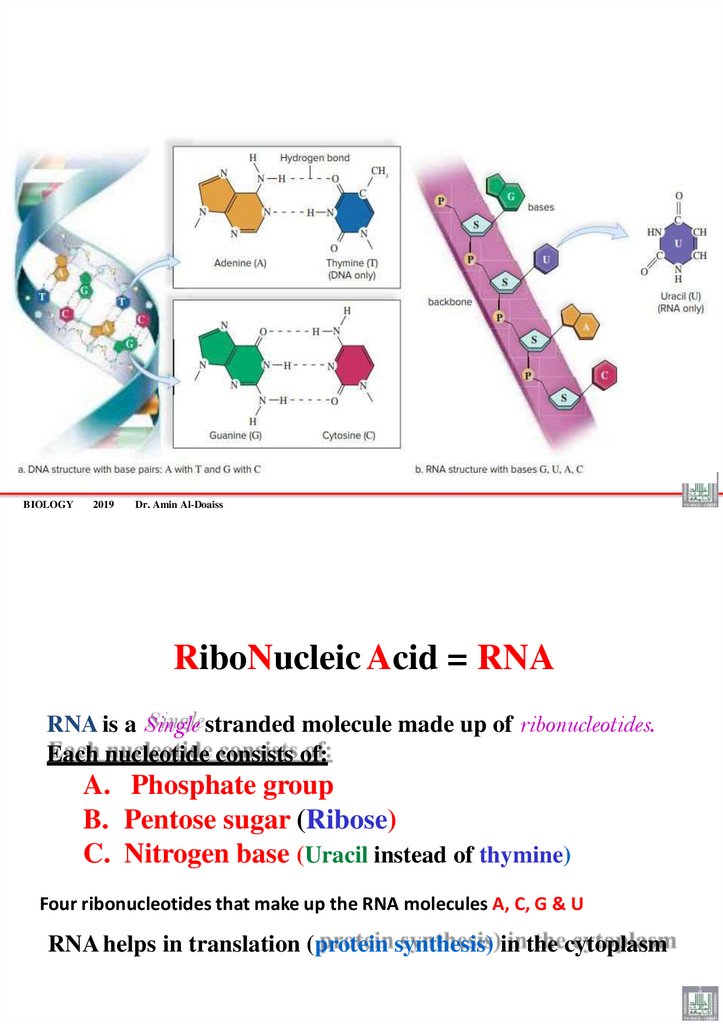
DNA Structure and FunctionThere are four different nitrogen bases in DNA:
• Adenine (A)
• Guanine (G)
Purine = 2 rings
• Thymine (T)
• Cytosine (C)
Pyrimidine= 1 ring
Base Pairing Rule
– Adenine (A) pairs with Thymine (T) or uracil (U) by two H-bonds.
– Guanine (G) pairs with Cytosine (C) by three H-bonds,
– G-C base-pairs are more stable than A-T base-pairs
A
T
(2 H-bonds)
G
BIOLOGY
2019
C
(3 H-bonds)
Dr. Amin Al-Doaiss
42.
BIOLOGY2019
Dr. Amin Al-Doaiss
RiboNucleic Acid = RNA
RNA is a Single stranded molecule made up of ribonucleotides.
Each nucleotide consists of:
A. Phosphate group
B. Pentose sugar (Ribose)
C. Nitrogen base (Uracil instead of thymine)
Four ribonucleotides that make up the RNA molecules A, C, G & U
RNA helps in translation (protein synthesis) in the cytoplasm
43.
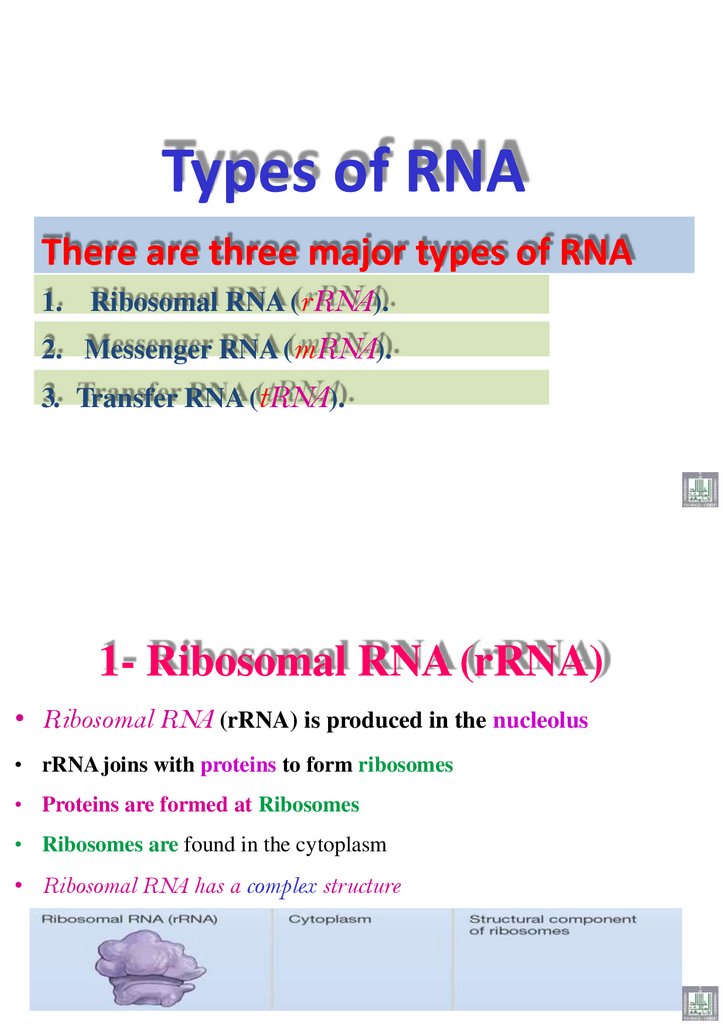
Types of RNAThere are three major types of RNA
1. Ribosomal RNA (rRNA).
2. Messenger RNA (mRNA).
3. Transfer RNA (tRNA).
1- Ribosomal RNA (rRNA)
• Ribosomal RNA (rRNA) is produced in the nucleolus
• rRNA joins with proteins to form ribosomes
• Proteins are formed at Ribosomes
• Ribosomes are found in the cytoplasm
• Ribosomal RNA has a complex structure
44.
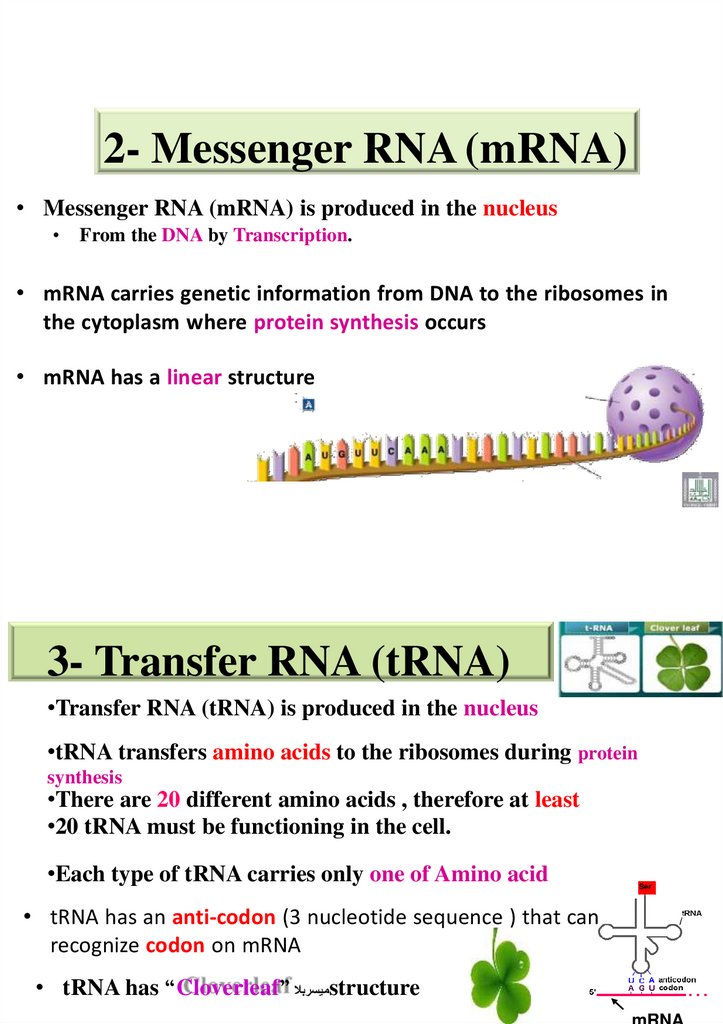
2- Messenger RNA (mRNA)• Messenger RNA (mRNA) is produced in the nucleus
From the DNA by Transcription.
• mRNA carries genetic information from DNA to the ribosomes in
the cytoplasm where protein synthesis occurs
• mRNA has a linear structure
3- Transfer RNA (tRNA)
•Transfer RNA (tRNA) is produced in the nucleus
•tRNA transfers amino acids to the ribosomes during protein
synthesis
•There are 20 different amino acids , therefore at least
•20 tRNA must be functioning in the cell.
•Each type of tRNA carries only one of Amino acid
• tRNA has an anti-codon (3 nucleotide sequence ) that can
recognize codon on mRNA
• tRNA has “Cloverleaf” ميسربالstructure
mRNA
45.
Codon-Anticodon base pairing• tRNA has Two ends:
A. Anticodon loop interacts with codon on the mRNA.
B. Amino acid attachment site at the opposite end
There are Different RNAs with Distinct Functions
BIOLOGY Copyright 2018 Dr. Amin Al-Doaiss
46.
DNA-RNA Similarities and differencesDr. AMIN ABDULLAH AL-DOAISS
BIOLOGY
2019
Dr. Amin Al-Doaiss
1
47.
BIOLOGY2019
Dr. Amin Al-Doaiss
2
THE CELL
• The term of “ cell” comes from the Latin word cella meaning
small room or “hut” خوك
• Cell is the smallest living unit of life
• Cell is the basic Structural, functional & biological unit of
living organisms
BIOLOGY
2019
Dr. Amin Al-Doaiss
3
48.
CELL THEORY• Cell theory refers to an idea that said: cells are the basic units of living things.
• Cell theory proposed by Schleiden, Schwann & Virchow
• The theory states that:
1. All living organisms are made up of one or more cells.
2. Cells are the basic units of life.
3. All cells arise only from pre-existing cells.
4. The cell is the unit of structure, function, and organization in living things.
BIOLOGY
2019
Dr. Amin Al-Doaiss
4
Cells are able to:
1 Reproduce
2 Grow and develop
3 Remain homeostasis
4 Respond to stimuli
5 Energy utilization & metabolism
6 Become adapted to the environment.
BIOLOGY
2019
Dr. Amin Al-Doaiss
5
12/20/144
49.
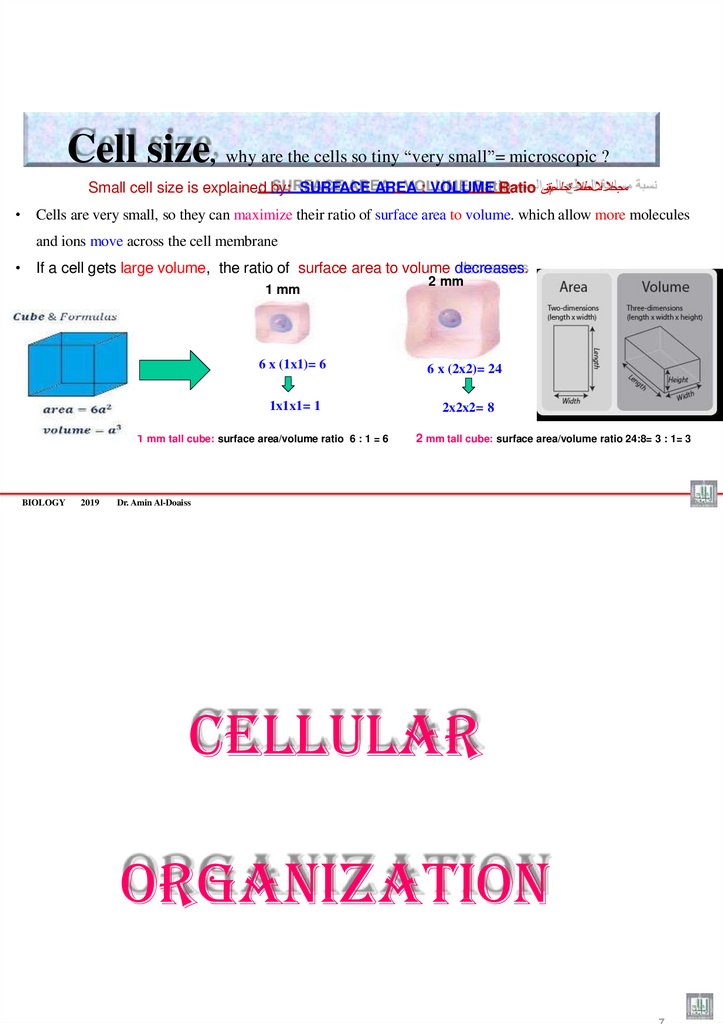
Cell size, why are the cells so tiny “very small”= microscopic ?Small cell size is explained by: SURFACE AREA : VOLUME Ratio مجحال ىالحطسال ةحاسمبةسن
• Cells are very small, so they can maximize their ratio of surface area to volume. which allow more molecules
and ions move across the cell membrane
• If a cell gets large volume, the ratio of surface area to volume decreases.
1 mm
6 x (1x1)= 6
6 x (2x2)= 24
1x1x1= 1
2x2x2= 8
1 mm tall cube: surface area/volume ratio 6 : 1 = 6
BIOLOGY
2019
2 mm
2 mm tall cube: surface area/volume ratio 24:8= 3 : 1= 3
6
Dr. Amin Al-Doaiss
CELLULAR
ORGANIZATION
7
50.
Cell Types• Cells can be classify into two categories:
1.
Prokaryotes (prokaryotic cells)
– Prokaryotic cells lack a nucleus
– Include two groups of bacteria (the bacteria and the archae)
2. Eukaryotes (Eukaryotic cells)
– Eukaryotic cells possess a nucleus
Organism Types
• There are two types:
BIOLOGY
1.
Unicellular = Single celled organism (one cell), NO tissues, organs, systems
2.
Multicellular (more than one cell)
2019
Dr. Amin Al-Doaiss
8
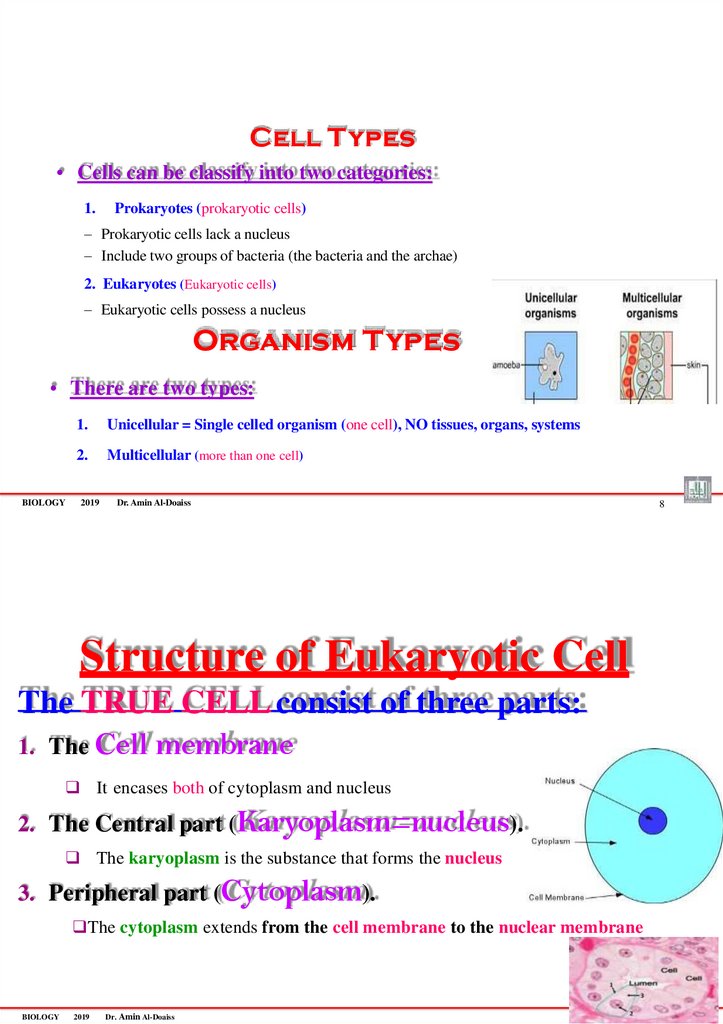
Structure of Eukaryotic Cell
The TRUE CELL consist of three parts:
1. The Cell membrane
It encases both of cytoplasm and nucleus
2. The Central part (Karyoplasm=nucleus).
The karyoplasm is the substance that forms the nucleus
3. Peripheral part (Cytoplasm).
The cytoplasm extends from the cell membrane to the nuclear membrane
9
BIOLOGY
2019
Dr. Amin Al-Doaiss
51.
BIOLOGY2019
Dr. Amin Al-Doaiss
10
1 Plasma membrane (cell membrane or plasmalemma)
1. Gives the cell its shape
2. Regulates movement of substances in and out of the cell.
3. Physical isolation between the cytoplasm and the external
environment.
2- Nucleus (brain of the cell = library of the cell)
• It is a large and centrally located structure containing chromosomes
• It is the control center of the cell.
• It has a nucleolus.
3. Cytoplasm
• It is a portion of the cell between plasma membrane and the nucleus.
• Cytoplasm is a semifluid or gel-like structure (due to proteins)
• Contains water and other suspended or dissolved molecules.
BIOLOGY
2019
Dr. Amin Al-Doaiss
11
52.
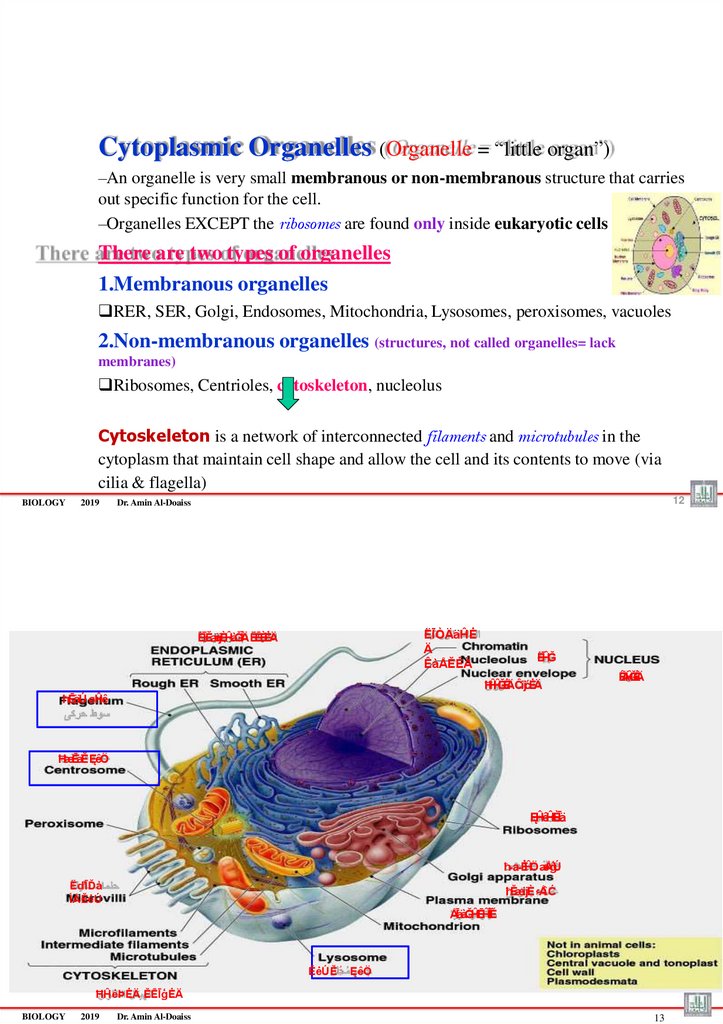
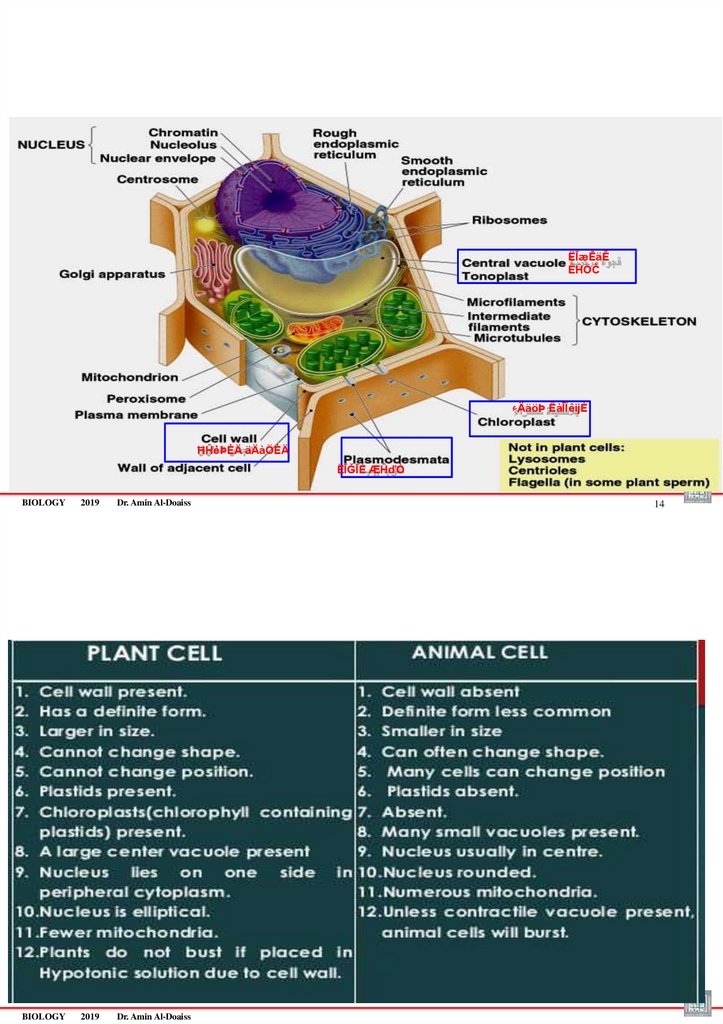
Cytoplasmic Organelles (Organelle = “little organ”)–An organelle is very small membranous or non-membranous structure that carries
out specific function for the cell.
–Organelles EXCEPT the ribosomes are found only inside eukaryotic cells
There are two types of organelles
1.Membranous organelles
RER, SER, Golgi, Endosomes, Mitochondria, Lysosomes, peroxisomes, vacuoles
2.Non-membranous organelles (structures, not called organelles= lack
membranes)
Ribosomes, Centrioles, cytoskeleton, nucleolus
Cytoskeleton is a network of interconnected filaments and microtubules in the
cytoplasm that maintain cell shape and allow the cell and its contents to move (via
cilia & flagella)
BIOLOGY
2019
12
Dr. Amin Al-Doaiss
ËĪÒÄäĤĖ
Ä
ÊàÅĚĖÄ
ËĪĚæijÈĤàĞİÄ ËĒÈîĖÄ
ËĪĤĞ
ĦĤĤĞĖÄĈijćĖÄ
ÊÄĤĞĖÄ
ħĒäÚ øĤê
ĦæĒäĚĘêÖ
ĘĤêĤÈĪä
ħـaـĖĤÖæÅģÚ
ËďĪĎà
ÌÅĚėÚ
ħĚæijÈ ءÅîĆ
ÅĪäàĞĤĒĤÎĪĚ
ĔėÚĚَ ĘêÖ
ĦĤėÞĖÄ ĔĒĪģĖÄ
BIOLOGY
2019
Dr. Amin Al-Doaiss
13
53.
ËĪæĒäĚÊĤÖĊ
ءÄäöÞ ÊàĪÎêijÈ
ĦĤėÞĖÄ äÄàÖĖÄ
ËĪĞĪÈ ÆĤďÒ
BIOLOGY
2019
Dr. Amin Al-Doaiss
BIOLOGY
2019
Dr. Amin Al-Doaiss
14
54.
BIOLOGY2019
Dr. Amin Al-Doaiss
16
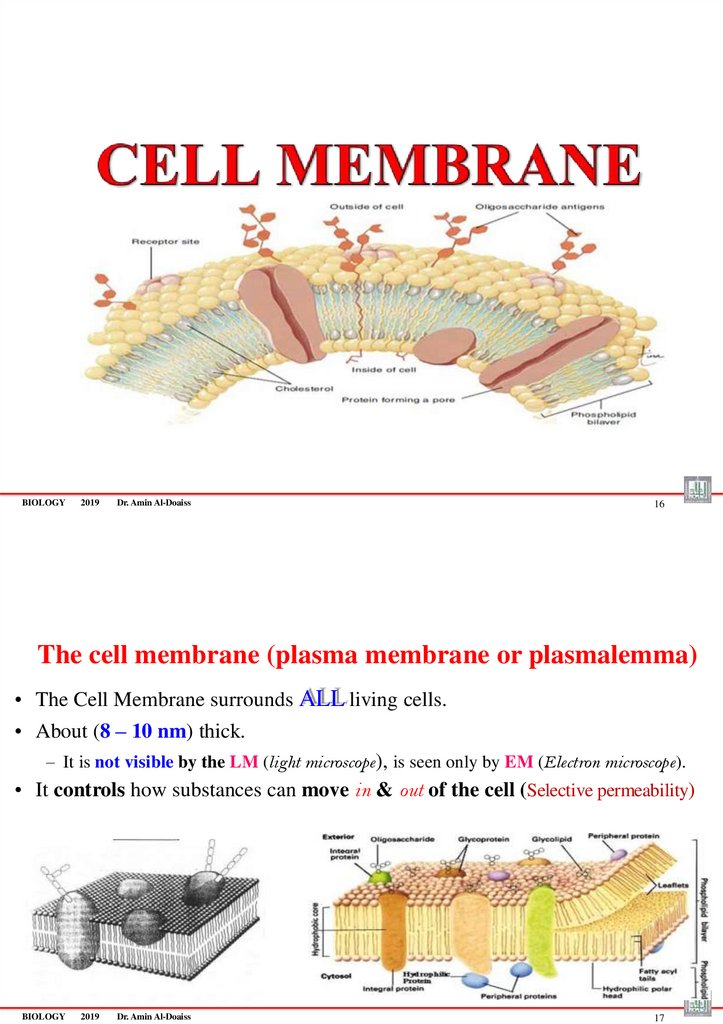
The cell membrane (plasma membrane or plasmalemma)
• The Cell Membrane surrounds ALL living cells.
• About (8 – 10 nm) thick.
– It is not visible by the LM (light microscope), is seen only by EM (Electron microscope).
• It controls how substances can move in & out of the cell (Selective permeability)
BIOLOGY
2019
Dr. Amin Al-Doaiss
17
55.
Structure Of Cell Membrane:Plasma Membrane consists of: Lipids,
as
a
proteins,
carbohydrates,
arranged
fluid mosaic model ĔÂÅêĖÄ ĩÂÅêċĪêċĖÄ ÔâĤĚĞĖÄ
“
Fluid refers to= movement of phospholipids molecules and proteins
Mosaic refers to=“Proteins “float” in the phospholipid bilayer, when viewed
from the top (Proteins like icebergs in a sea of lipids)
BIOLOGY
2019
Dr. Amin Al-Doaiss
18
1. Lipids
Phospholipids: bilayer
Cholesterol: limits and regulates the membrane fluidity.
2. Protein
– Peripheral proteins: attached to either the inner or outer membrane surface.
– Integral proteins: penetrate both Phospholipid bilayer to form channels
3. carbohydrates (Glycoproteins & glycolipids): act as receptors, antigens
BIOLOGY
2019
Dr. Amin Al-Doaiss
19
56.
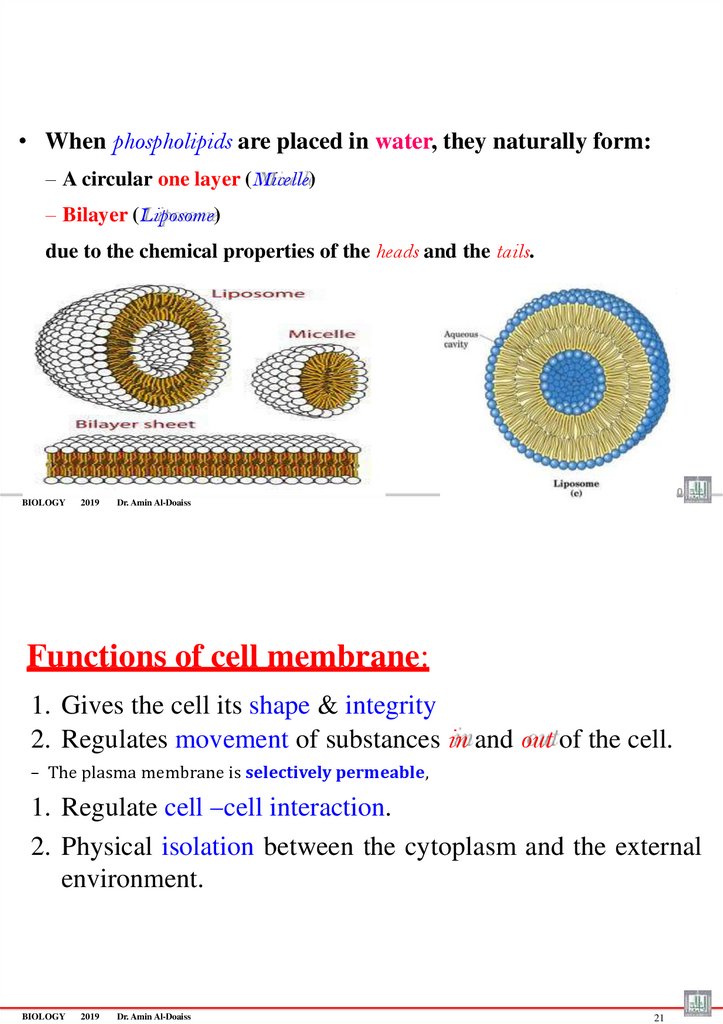
• When phospholipids are placed in water, they naturally form:– A circular one layer (Micelle)
– Bilayer (Liposome)
due to the chemical properties of the heads and the tails.
2
0
BIOLOGY
2019
Dr. Amin Al-Doaiss
Functions of cell membrane:
1. Gives the cell its shape & integrity
2. Regulates movement of substances in and out of the cell.
– The plasma membrane is selectively permeable,
1. Regulate cell –cell interaction.
2. Physical isolation between the cytoplasm and the external
environment.
BIOLOGY
2019
Dr. Amin Al-Doaiss
21
57.
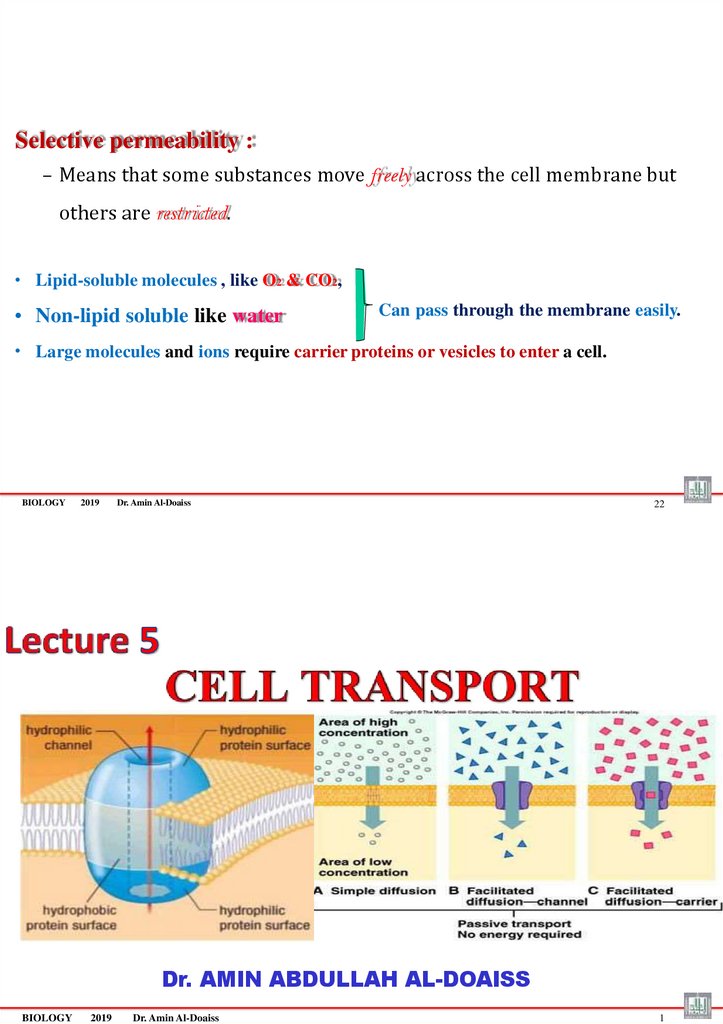
Selective permeability :– Means that some substances move freely across the cell membrane but
others are restricted.
• Lipid-soluble molecules , like O2 & CO2,
• Non-lipid soluble like water
Can pass through the membrane easily.
• Large molecules and ions require carrier proteins or vesicles to enter a cell.
BIOLOGY
2019
Dr. Amin Al-Doaiss
22
Dr. AMIN ABDULLAH AL-DOAISS
BIOLOGY
2019
Dr. Amin Al-Doaiss
1
58.
Cell Transport is moving materials into, out of, or within the cell•The plasma membrane regulates the transport of substances into and out of the
cell.
•The plasma membrane is selectively permeable,
which means that some substances move freely across the membrane but others
are restricted.
CO2
Nucleus
O2
BIOLOGY
2019
Dr. Amin Al-Doaiss
2
Cell Transport done by TWO ways
1. Passive transport لمالخالقنال
2. Active transport طشناللقنال
BIOLOGY
2019
Dr. Amin Al-Doaiss
3
59.
BIOLOGY2019
Dr. Amin Al-Doaiss
4
1
Passive transport
(No Energy Required) )َلماخال لقنال (بلسال
BIOLOGY
2019
Dr. Amin Al-Doaiss
5
60.
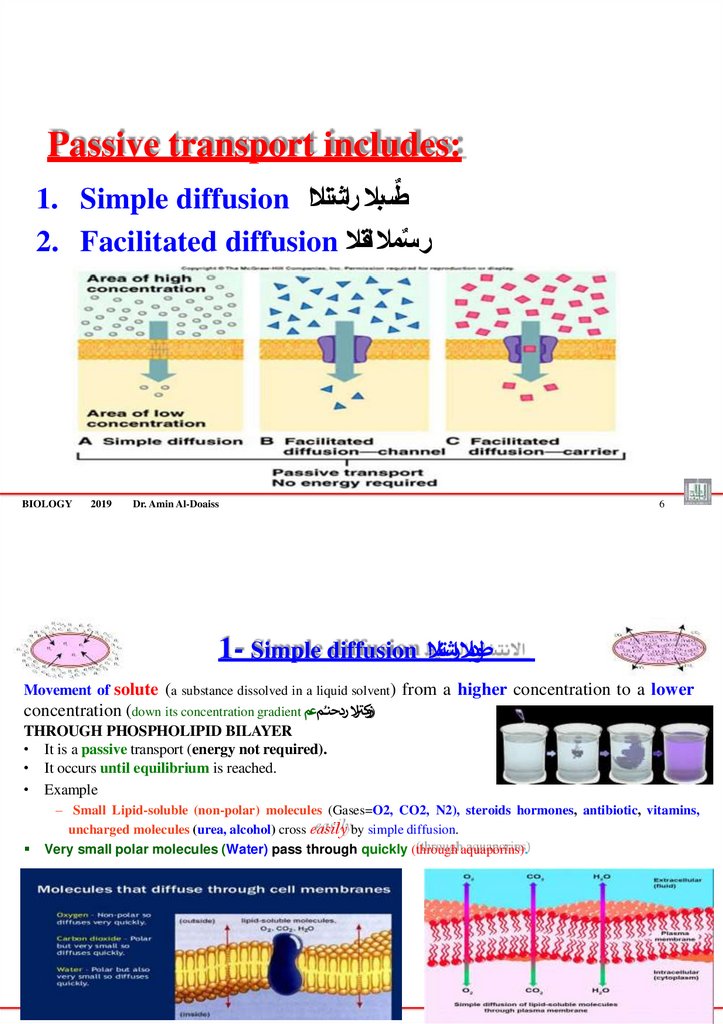
Passive transport includes:ٌ
1. Simple diffusion طسبال راشتنالا
2. Facilitated diffusion سمال لقنال
ٌ ر
BIOLOGY
2019
Dr. Amin Al-Doaiss
1- Simple diffusion
6
طودبال ارشتنالا
Movement of solute (a substance dissolved in a liquid solvent) from a higher concentration to a lower
)زو ر
concentration (down its concentration gradient كتال ردحنـُمعم
THROUGH PHOSPHOLIPID BILAYER
• It is a passive transport (energy not required).
• It occurs until equilibrium is reached.
• Example
– Small Lipid-soluble (non-polar) molecules (Gases=O2, CO2, N2), steroids hormones, antibiotic, vitamins,
uncharged molecules (urea, alcohol) cross easily by simple diffusion.
Very small polar molecules (Water) pass through quickly (through aquaporins).
BIOLOGY
2019
Dr. Amin Al-Doaiss
7
61.
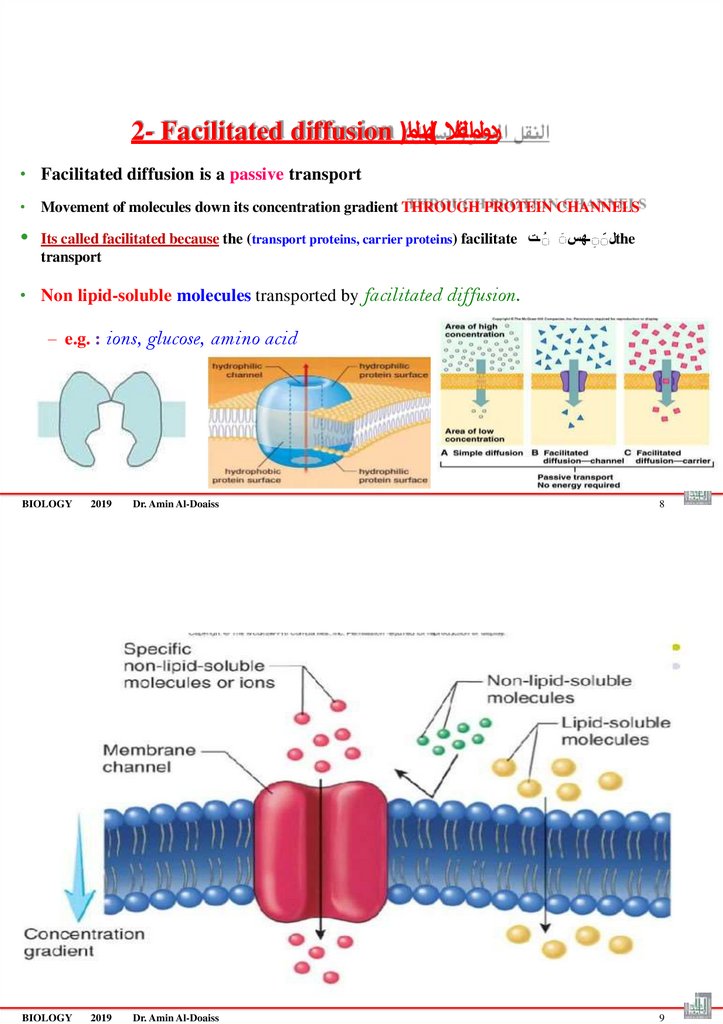
2- Facilitated diffusion )ردولمالقنال (لهدل ام• Facilitated diffusion is a passive transport
• Movement of molecules down its concentration gradient THROUGH PROTEIN CHANNELS
Its called facilitated because the (transport proteins, carrier proteins) facilitate لََـهسَ َـتthe
transport
• Non lipid-soluble molecules transported by facilitated diffusion.
– e.g. : ions, glucose, amino acid
BIOLOGY
2019
Dr. Amin Al-Doaiss
8
BIOLOGY
2019
Dr. Amin Al-Doaiss
9
62.
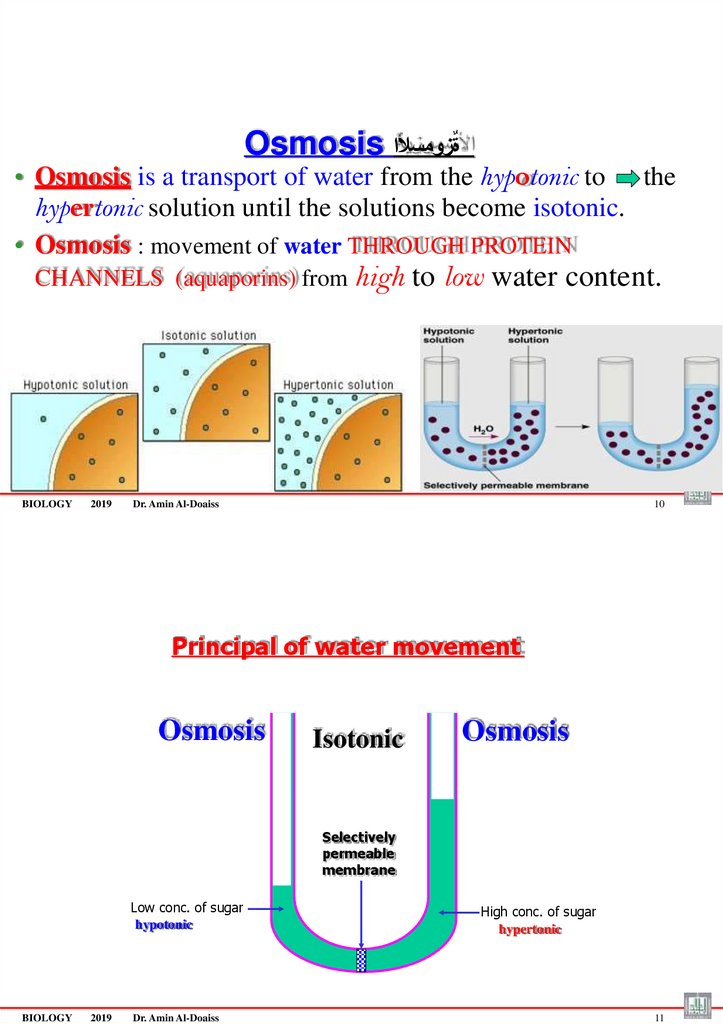
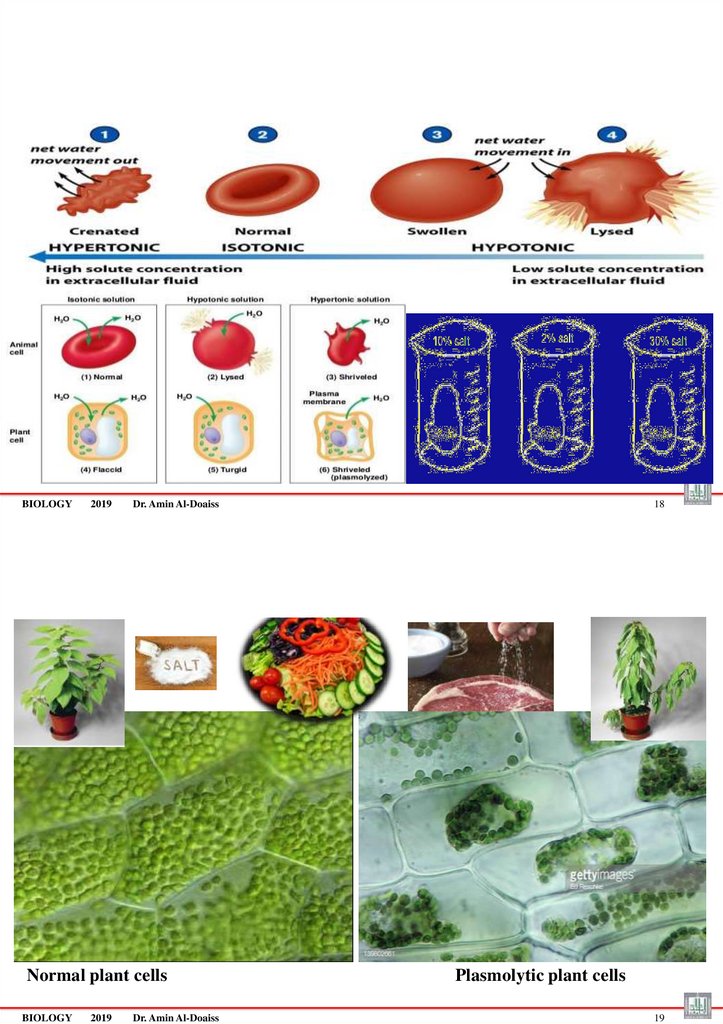
Osmosis ةٌزومسألا• Osmosis is a transport of water from the hypotonic to the
hypertonic solution until the solutions become isotonic.
• Osmosis : movement of water THROUGH PROTEIN
CHANNELS (aquaporins) from high
BIOLOGY
2019
to low water content.
Dr. Amin Al-Doaiss
10
Principal of water movement
Osmosis
Isotonic
Osmosis
Selectively
permeable
membrane
Low conc. of sugar
hypotonic
BIOLOGY
2019
Dr. Amin Al-Doaiss
High conc. of sugar
hypertonic
11
63.
Tonicity• Hyper = above
• Iso
= same
• Hypo = below
• Tonicity: comparing the concentration of solute in two solutions.
• Example: the concentration of sugar or salt in two solutions
The solutions are divided according to tonicity into:
• Isotonic: لداعتم
• Hypertonic
same solute concentration outside of the cell
ٌزكرتال ىالعhigher concentration of solutes outside of the cell
• Hypotonic: ٌزكرتال ضفخنمlower concentration of solutes outside of the cell
BIOLOGY
2019
Solutes
= water
Solutes more than water
Solutes less than water
Dr. Amin Al-Doaiss
12
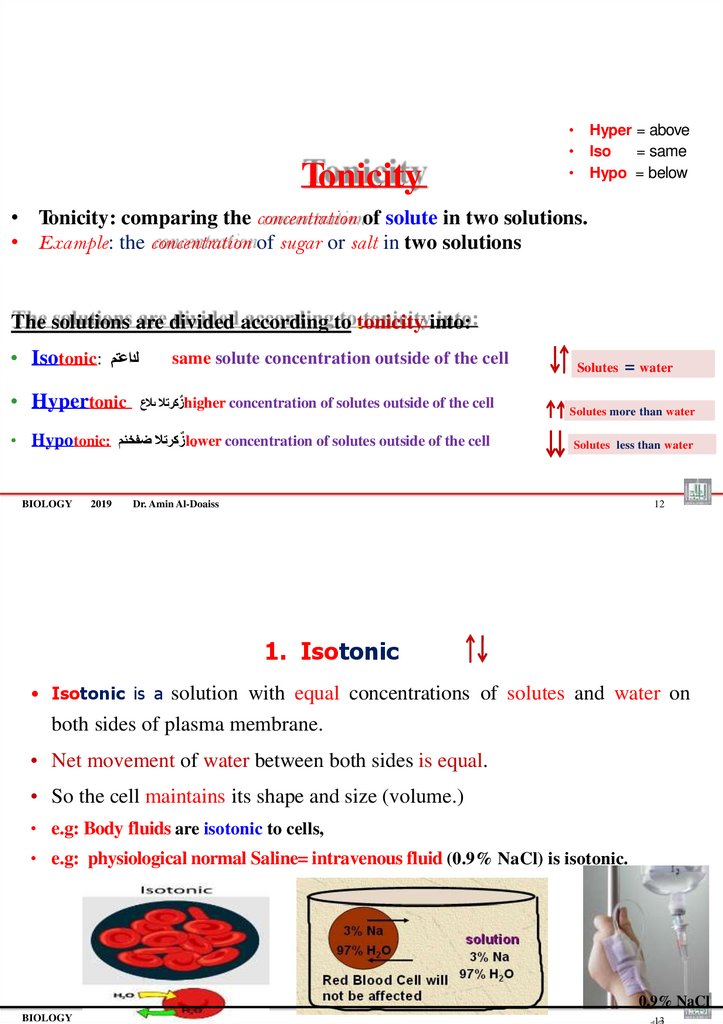
1. Isotonic
• Isotonic is a solution with equal concentrations of solutes and water on
both sides of plasma membrane.
• Net movement of water between both sides is equal.
• So the cell maintains its shape and size (volume.)
• e.g: Body fluids are isotonic to cells,
• e.g: physiological normal Saline= intravenous fluid (0.9% NaCl) is isotonic.
2019
Dr. Amin Al-Doaiss
0.9% NaCl
BIOLOGY
13
64.
2. Hypotonic•Hypotonic
solution
solute
concentration
water concentration.
has
and
lower
higher
•So water flows (diffuses) inward the cell, from
high concentration of water to low (within the cell)
.
•So cell swells and bursts.
•e.g: distilled water (pure water)
BIOLOGY
2019
14
Dr. Amin Al-Doaiss
3. Hypertonic
• A hypertonic solution has higher solute concentration and lower concentration of
water, such as in very brackish (salty) water .
• So water flows (diffuse) out of the cell, from high concentration of water (within the
cell) to low con. outside the cell.
• So much water leaves the cell and shrinks or shrivels.
• Red blood cells will shrink and shrivel (crenation)
• e.g: sea water
BIOLOGY
2019
Dr. Amin Al-Doaiss
15
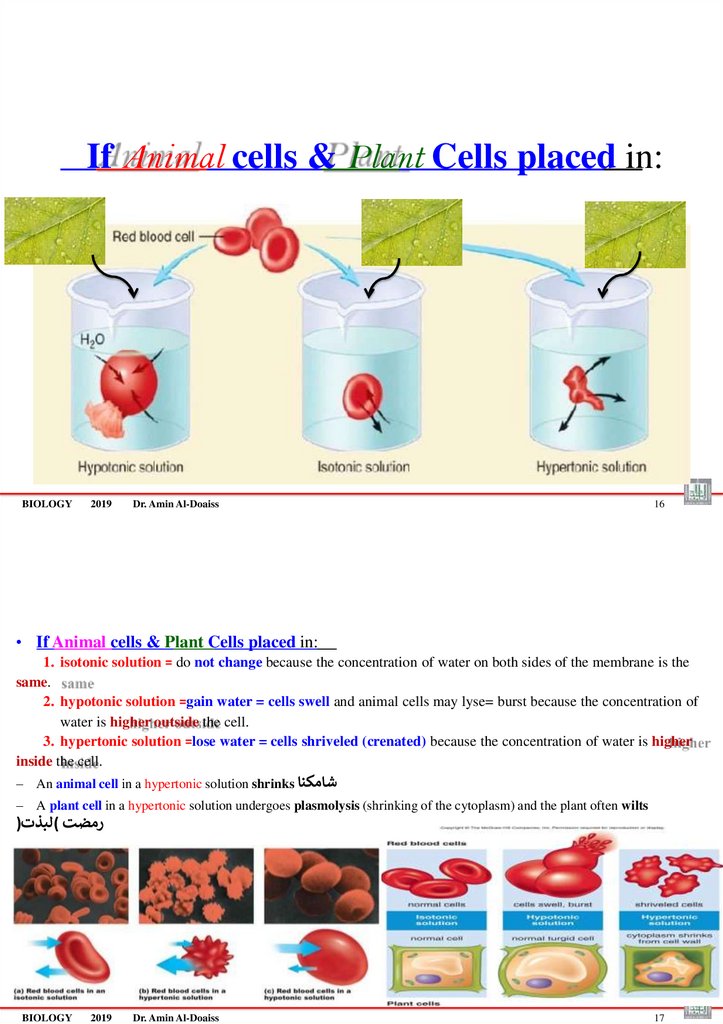
65. If Animal cells & Plant Cells placed in:
If Animal cells & Plant Cells placed in:BIOLOGY
2019
Dr. Amin Al-Doaiss
16
• If Animal cells & Plant Cells placed in:
1. isotonic solution = do not change because the concentration of water on both sides of the membrane is the
same.
2. hypotonic solution =gain water = cells swell and animal cells may lyse= burst because the concentration of
water is higher outside the cell.
3. hypertonic solution =lose water = cells shriveled (crenated) because the concentration of water is higher
inside the cell.
–
An animal cell in a hypertonic solution shrinks شامكنا
–
A plant cell in a hypertonic solution undergoes plasmolysis (shrinking of the cytoplasm) and the plant often wilts
)رمضت (لبذت
BIOLOGY
2019
Dr. Amin Al-Doaiss
17
66.
BIOLOGY2019
Dr. Amin Al-Doaiss
Normal plant cells
BIOLOGY
2019
Dr. Amin Al-Doaiss
18
Plasmolytic plant cells
19
67.
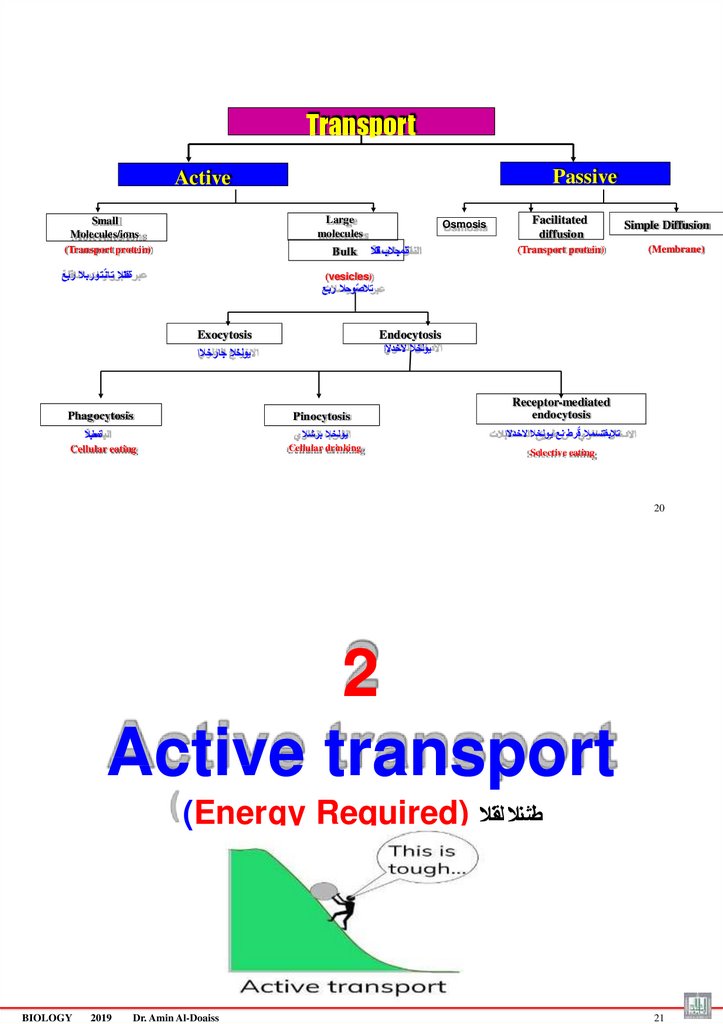
TransportPassive
Active
Large
molecules
Small
Molecules/ions
(Transport protein)
Bulk
ةلقانال ت ان ٌتوربال ربع
Osmosis
ةلمجالب لقنال
Facilitated
diffusion
(Transport protein)
Simple Diffusion
(Membrane)
(vesicles)
تالصٌوحال ربع
Exocytosis
Endocytosis
يولخال الخدالا
يولخال جارخالا
Phagocytosis
Pinocytosis
ةمعلبال
Cellular eating
Receptor-mediated
endocytosis
يولخال برشال
تالبقتسمال قٌرط نع يولخال الخدالا
Cellular drinking
Selective eating
20
2
Active transport
(Energy Required) طشنال لقنال
BIOLOGY
2019
Dr. Amin Al-Doaiss
21
68.
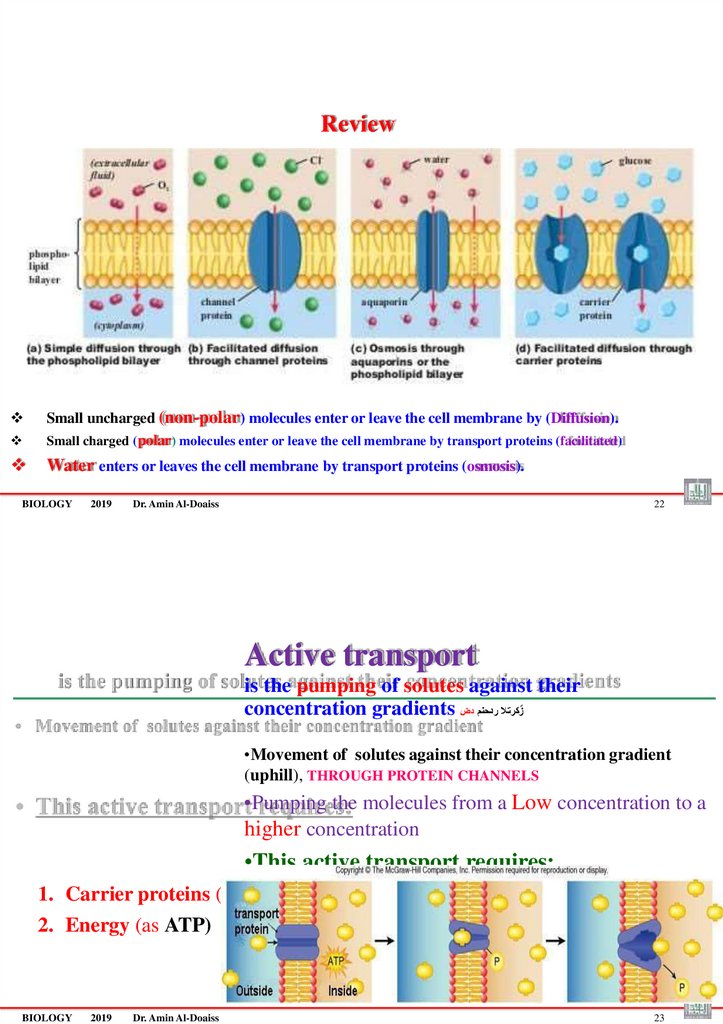
ReviewSmall uncharged (non-polar) molecules enter or leave the cell membrane by (Diffusion).
Small charged (polar) molecules enter or leave the cell membrane by transport proteins (facilitated)
Water enters or leaves the cell membrane by transport proteins (osmosis).
BIOLOGY
2019
Dr. Amin Al-Doaiss
22
Active transport
is the pumping of solutes against their
concentration gradients زٌ كرتال ردحنم دض
•Movement of solutes against their concentration gradient
(uphill), THROUGH PROTEIN CHANNELS
•Pumping the molecules from a Low concentration to a
higher concentration
•This active transport requires:
1. Carrier proteins (pumps)
2. Energy (as ATP)
BIOLOGY
2019
Dr. Amin Al-Doaiss
23
69.
TransportPassive
Active
Small
Molecules/ions
Large
molecules
(Transport protein)
(vesicles)
= Bulk
ةلمجالب لقنال
تالصٌوحال ربع
ةلقانال ت ان ٌتوربال ربع
Exocytosis
Endocytosis
يولخال جارخالا
يولخال الخدالا
Receptor-mediated
BIOLOGY
Phagocytosis
Pinocytosis
endocytosis
يولخال لكالا
يولخال برشال
تالبقتسمال قٌرط نع يولخال الخدالا
Cellular eating
Cellular drinking
Selective eating
2019
Dr. Amin Al-Doaiss
24
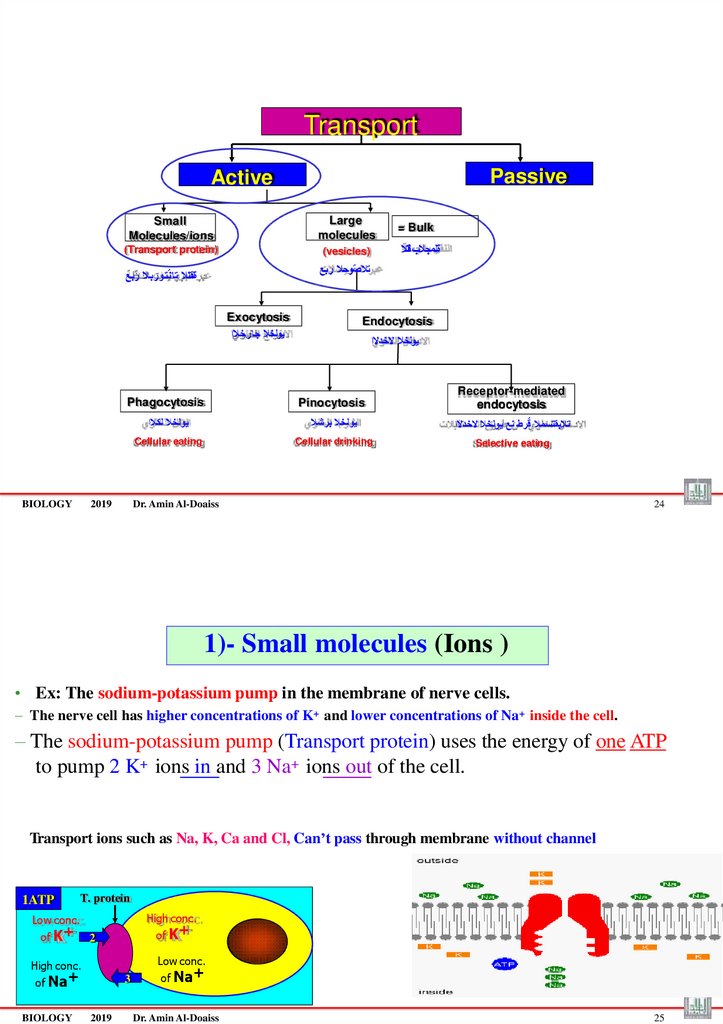
1)- Small molecules (Ions )
• Ex: The sodium-potassium pump in the membrane of nerve cells.
– The nerve cell has higher concentrations of K+ and lower concentrations of Na+ inside the cell.
– The sodium-potassium pump (Transport protein) uses the energy of one ATP
to pump 2 K+ ions in and 3 Na+ ions out of the cell.
Transport ions such as Na, K, Ca and Cl, Can’t pass through membrane without channel
1ATP
T. protein
High conc.
Low conc.
of K+
of K+
2
Low conc.
High conc.
of Na+
BIOLOGY
3
2019
of Na+
Dr. Amin Al-Doaiss
25
70.
BIOLOGY2019
Dr. Amin Al-Doaiss
26
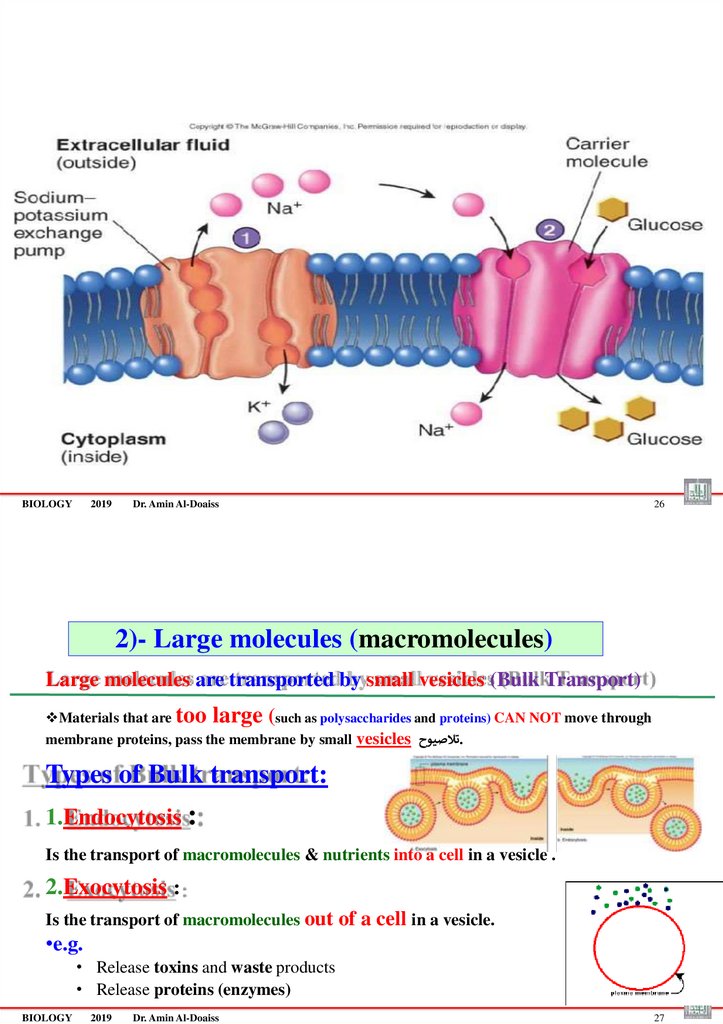
2)- Large molecules (macromolecules)
Large molecules are transported by small vesicles (Bulk Transport)
Materials that are too large (such as polysaccharides and proteins) CAN NOT move through
membrane proteins, pass the membrane by small vesicles تالصيوح.
Types of Bulk transport:
1.Endocytosis :
Is the transport of macromolecules & nutrients into a cell in a vesicle .
2.Exocytosis :
Is the transport of macromolecules out of a cell in a vesicle.
•e.g.
• Release toxins and waste products
• Release proteins (enzymes)
BIOLOGY
2019
Dr. Amin Al-Doaiss
27
71.
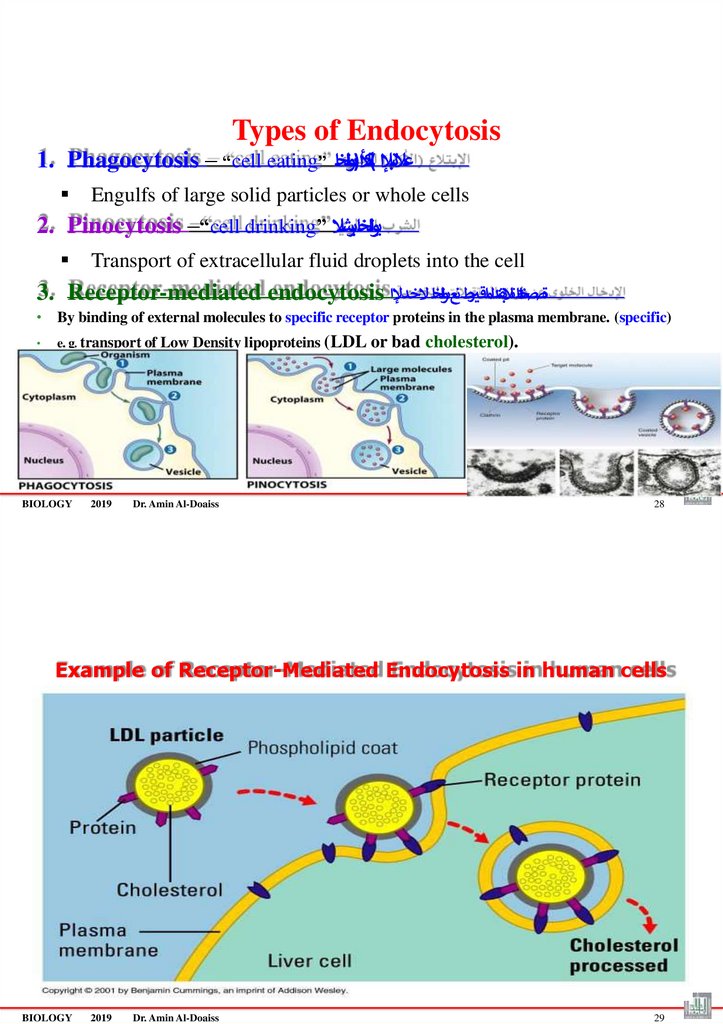
Types of Endocytosis1. Phagocytosis – “cell eating” أل)ىولخا
التبإلا (لك ا
ُع
Engulfs of large solid particles or whole cells
2. Pinocytosis –“cell drinking” يولخابرشال
Transport of extracellular fluid droplets into the cell
3. Receptor-mediated endocytosis ةصصختلماتالبقتدلما رقتطنعىولخا ُالخدإلا
• By binding of external molecules to specific receptor proteins in the plasma membrane. (specific)
e. g. transport of Low Density lipoproteins (LDL or
BIOLOGY
2019
Dr. Amin Al-Doaiss
bad cholesterol).
28
Example of Receptor-Mediated Endocytosis in human cells
BIOLOGY
2019
Dr. Amin Al-Doaiss
29
72.
Dr. Amin Al-DoaissBIOLOGY
2019
Dr. Amin Al-Doaiss
1
1-The nucleus
BIOLOGY
2019
Dr. Amin Al-Doaiss
2
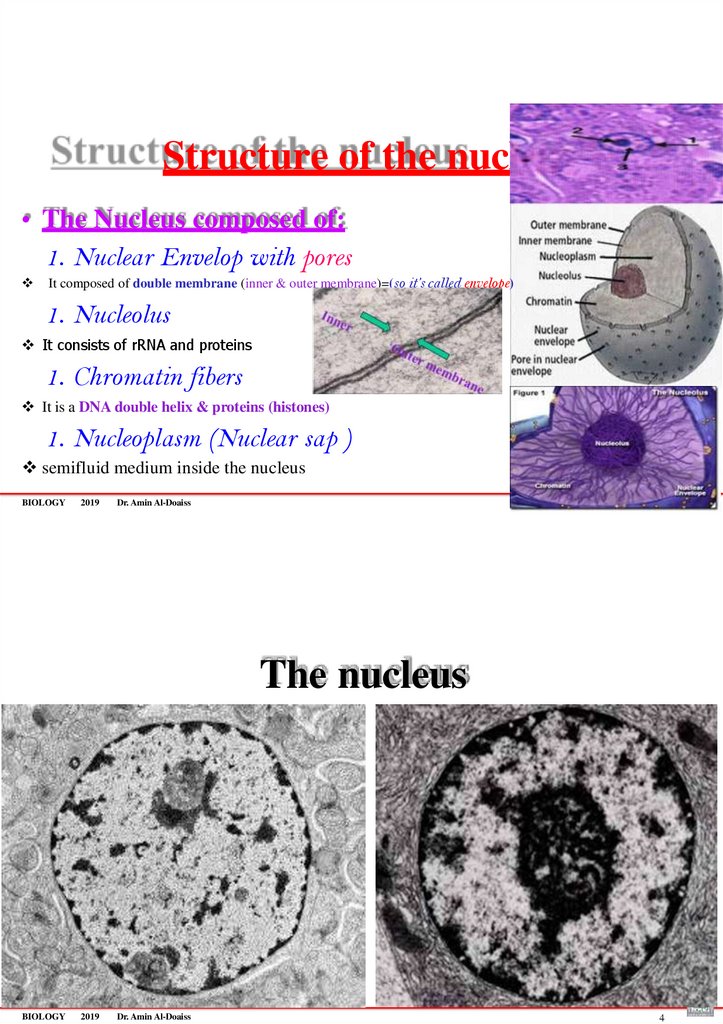
73. Structure of the nucleus
• The Nucleus composed of:1. Nuclear Envelop with pores
It composed of double membrane (inner & outer membrane)=(so it’s called envelope)
1. Nucleolus
It consists of rRNA and proteins
1. Chromatin fibers
3
It is a DNA double helix & proteins (histones)
1. Nucleoplasm (Nuclear sap )
semifluid medium inside the nucleus
BIOLOGY
2019
Dr. Amin Al-Doaiss
The nucleus
BIOLOGY
2019
Dr. Amin Al-Doaiss
4
74.
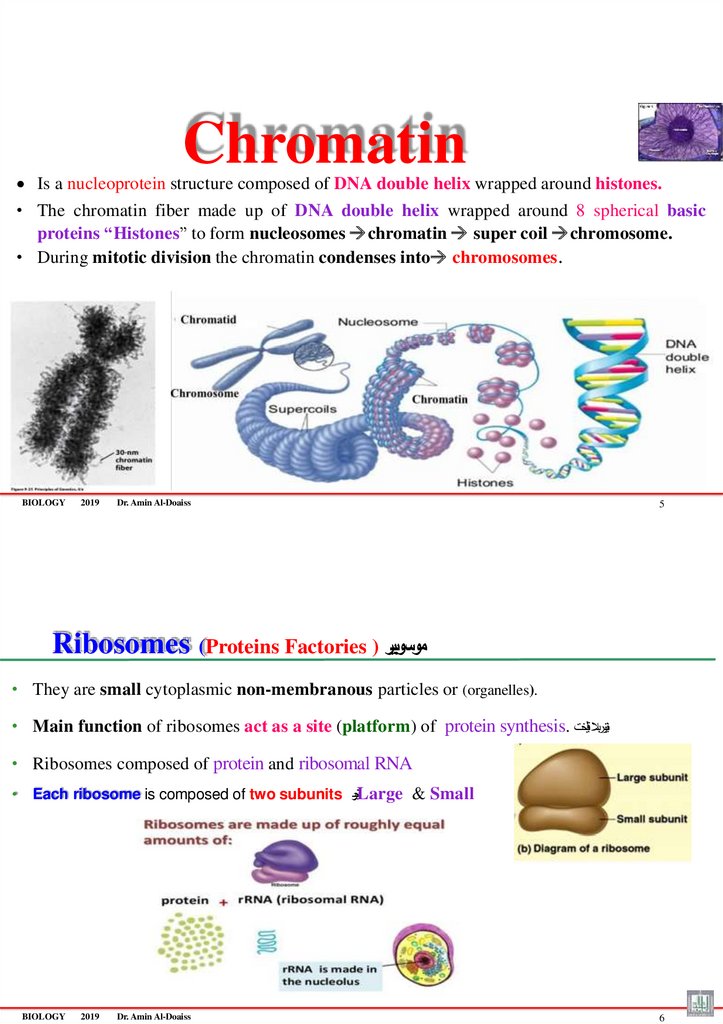
ChromatinIs a nucleoprotein structure composed of DNA double helix wrapped around histones.
• The chromatin fiber made up of DNA double helix wrapped around 8 spherical basic
proteins “Histones” to form nucleosomes chromatin super coil chromosome.
• During mitotic division the chromatin condenses into chromosomes.
BIOLOGY
2019
Dr. Amin Al-Doaiss
5
Ribosomes (Proteins Factories ) موسوبير
• They are small cytoplasmic non-membranous particles or (organelles).
• Main function of ribosomes act as a site (platform) of protein synthesis. نيتوربال قيلخت
• Ribosomes composed of protein and ribosomal RNA
• Each ribosome is composed of two subunits تيندحوLarge & Small
BIOLOGY
2019
Dr. Amin Al-Doaiss
6
75.
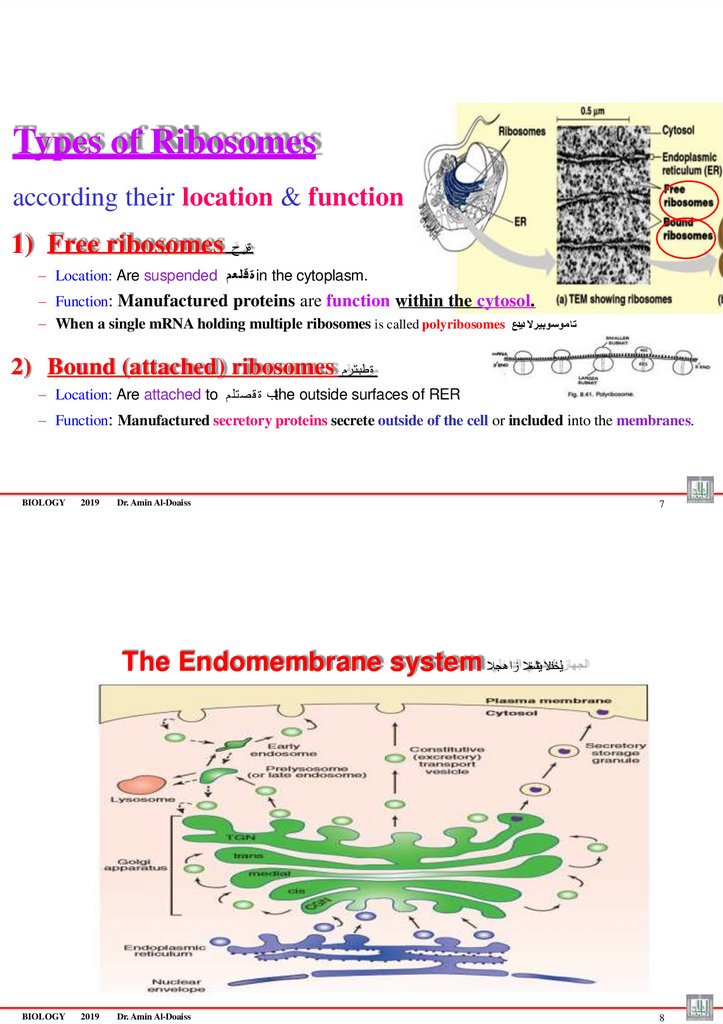
Types of Ribosomesaccording their location & function
1) Free ribosomes
ةرح
– Location: Are suspended ة قلع مin the cytoplasm.
– Function: Manufactured proteins are function within the cytosol.
– When a single mRNA holding multiple ribosomes is called polyribosomes ت ا موسوبيرال ديدع
2) Bound (attached) ribosomes
ة طبت رم
– Location: Are attached to ـب ة ق صت لمthe outside surfaces of RER
– Function: Manufactured secretory proteins secrete outside of the cell or included into the membranes.
BIOLOGY
2019
Dr. Amin Al-Doaiss
7
The Endomembrane system يلخادال يئاشغال زاهجال
BIOLOGY
2019
Dr. Amin Al-Doaiss
8
76.
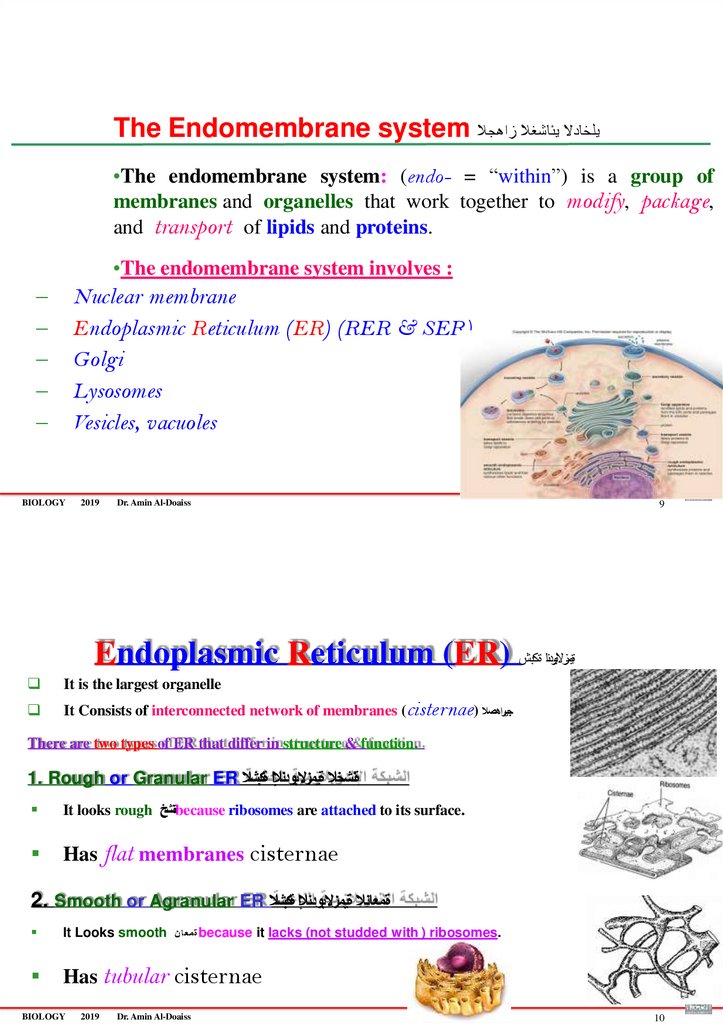
The Endomembrane system يلخادال يئاشغال زاهجال•The endomembrane system: (endo- = “within”) is a group of
membranes and organelles that work together to modify, package,
and transport of lipids and proteins.
•The endomembrane system involves :
–
–
–
–
–
Nuclear membrane
Endoplasmic Reticulum (ER) (RER & SER)
Golgi
Lysosomes
Vesicles, vacuoles
BIOLOGY
2019
Dr. Amin Al-Doaiss
9
Endoplasmic Reticulum (ER) ةيمزالبودنا ةكبش
It is the largest organelle
It Consists of interconnected network of membranes (cisternae) جيراهصال
There are two types of ER that differ in structure & function.
1. Rough or Granular ER ةنشخال ةيمزالبودنإلا ةكبشال
It looks rough ةنشخbecause ribosomes are attached to its surface.
Has flat membranes cisternae
2. Smooth or Agranular ER ةمعانال ةيمزالبودنإلا ةكبشال
It Looks smooth
Has tubular cisternae
BIOLOGY
2019
ة م ع ا نbecause
Dr. Amin Al-Doaiss
it lacks (not studded with ) ribosomes.
10
77.
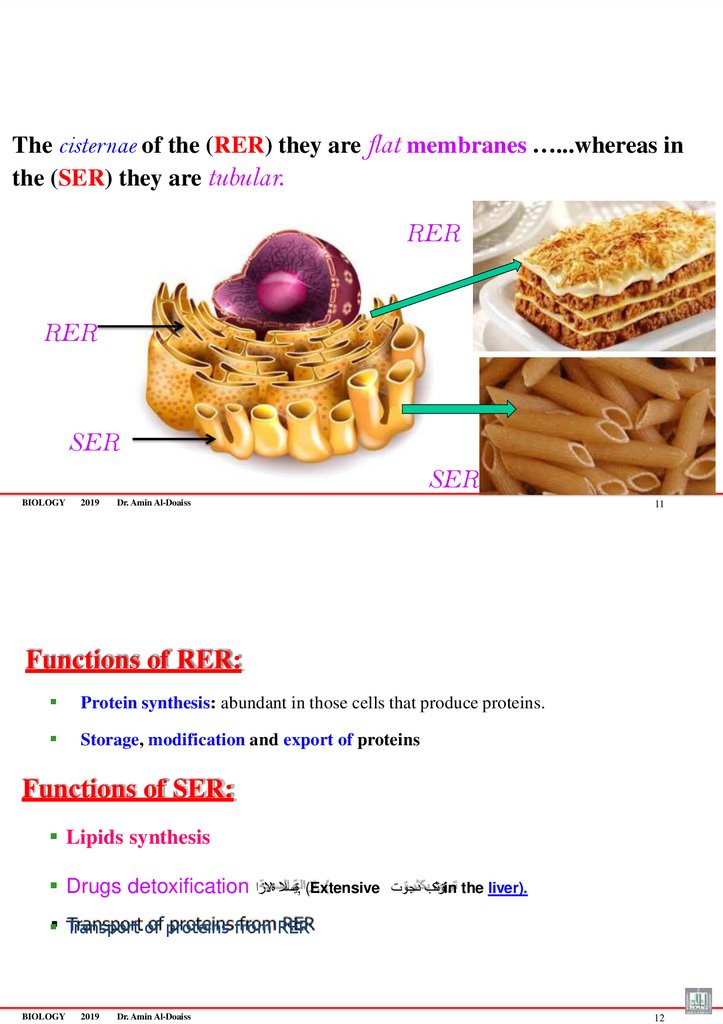
The cisternae of the (RER) they are flat membranes …...whereas inthe (SER) they are tubular.
RER
RER
SER
SER
BIOLOGY
2019
Dr. Amin Al-Doaiss
11
Functions of RER:
Protein synthesis: abundant in those cells that produce proteins.
Storage, modification and export of proteins
Functions of SER:
Lipids synthesis
Drugs detoxification ةيمسال ةالزا, (Extensive
ةرثكب دجوتin the liver).
Transport of proteins from RER
BIOLOGY
2019
Dr. Amin Al-Doaiss
12
78.
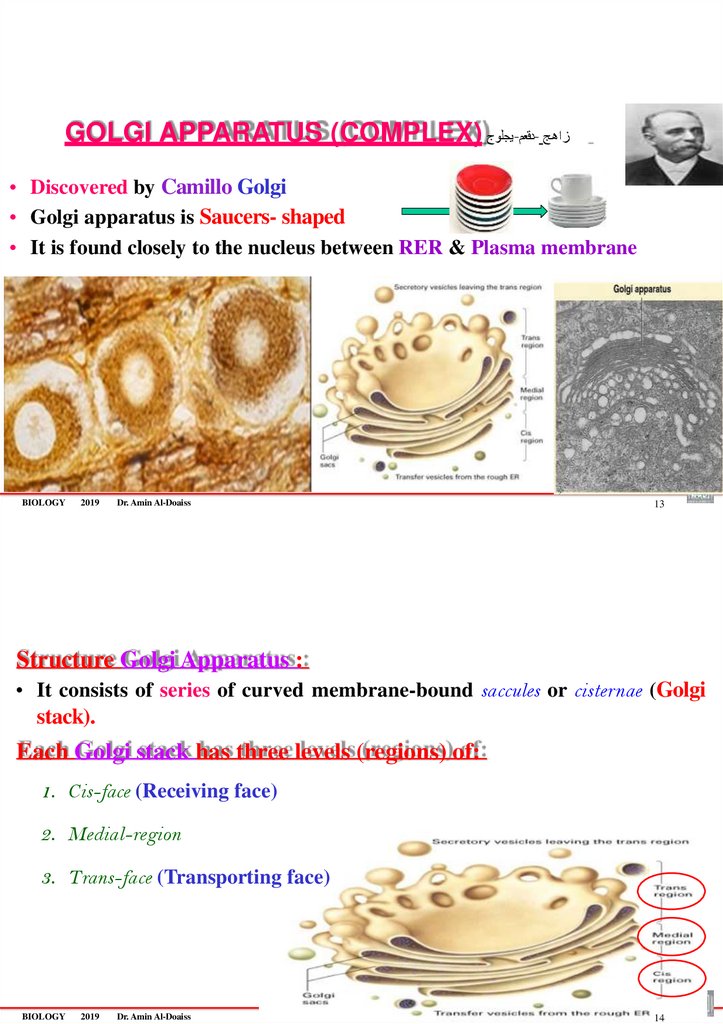
GOLGI APPARATUS (COMPLEX) يجلوج-دقعم- زاهج• Discovered by Camillo Golgi
• Golgi apparatus is Saucers- shaped
• It is found closely to the nucleus between RER & Plasma membrane
BIOLOGY
2019
Dr. Amin Al-Doaiss
13
Structure Golgi Apparatus :
• It consists of series of curved membrane-bound saccules or cisternae (Golgi
stack).
Each Golgi stack has three levels (regions) of:
1. Cis-face (Receiving face)
2. Medial-region
3. Trans-face (Transporting face)
BIOLOGY
2019
Dr. Amin Al-Doaiss
14
79.
Function of Golgi apparatus• Manufacturing,
• Packaging,
• Sorting,
• Modifications, Concentration
• shipping لقن
– of secretory products (proteins, lipids, carbohydrates) to outside the cell (secretion).
• Formation of vesicles and lysosomes.
BIOLOGY
2019
Dr. Amin Al-Doaiss
15
Pathway of the secretion
•The transport vesicles are come from the ER
and bring proteins/lipids to the Golgi
apparatus where they are modified
and packaged as secretory vesicles.
•Secretory vesicles fuse with plasma
membrane by exocytosis
during secretion to release the secretory
products.
Hormones/Enzymes Secretion Pathway:
ER→ Golgi → vesicles → plasma membrane
BIOLOGY
2019
Dr. Amin Al-Doaiss
16
80.
Cellular GarbageTHE LYSOSOME
Digestive bodies
ة لحمُال ماسجألا
Trash collector of the cell
BIOLOGY
2019
Dr. Amin Al-Doaiss
17
LYSOSOMES
• In Greek lysis= solution, soma= body (recycling center)
• Small membranous sacs or vesicles
• Contain more than 40 hydrolytic enzymes (acid hydrolases)
– using water to split chemical bond
• Originate from the Golgi apparatus.
• Work best at acidic pH (optimal pH= 5).
• Lysosomes play a role in DIGESTION of:
– Macromolecules, cellular debris and old organelles
– Particles come from outside of the cell
BIOLOGY
2019
Dr. Amin Al-Doaiss
18
81. Energy-Related Organelles
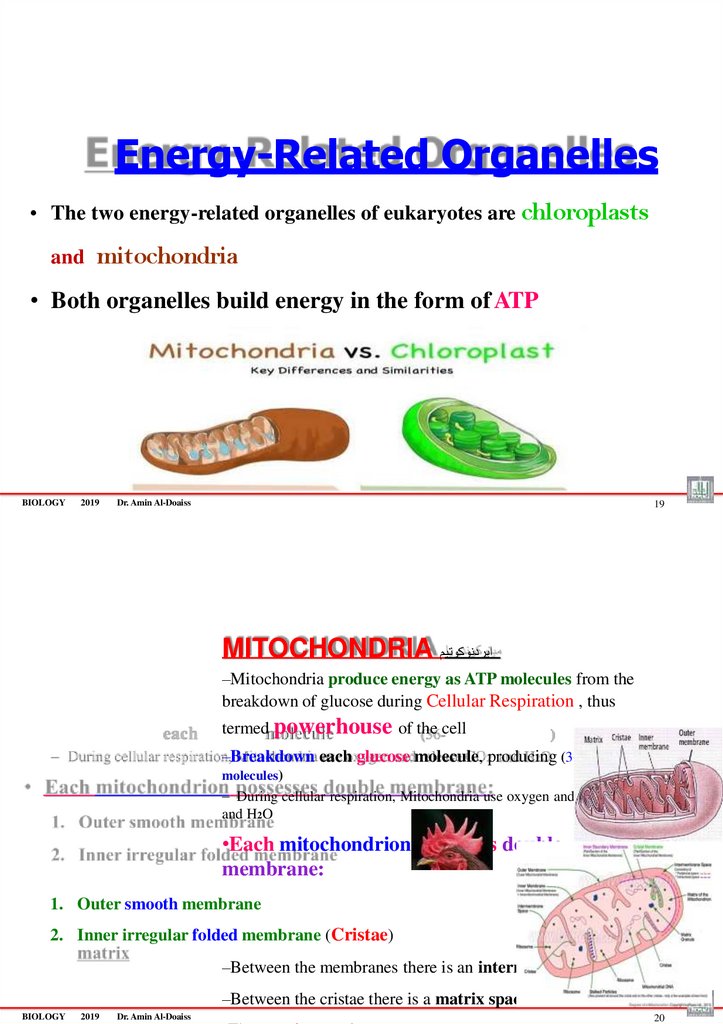
• The two energy-related organelles of eukaryotes are chloroplastsand
mitochondria
• Both organelles build energy in the form of ATP
BIOLOGY
2019
Dr. Amin Al-Doaiss
19
MITOCHONDRIA ايردنوكوتيم
–Mitochondria produce energy as ATP molecules from the
breakdown of glucose during Cellular Respiration , thus
termed powerhouse of the cell
–Breakdown each glucose molecule, producing (36-38 ATP
molecules)
– During cellular respiration, Mitochondria use oxygen and release CO2
and H2O
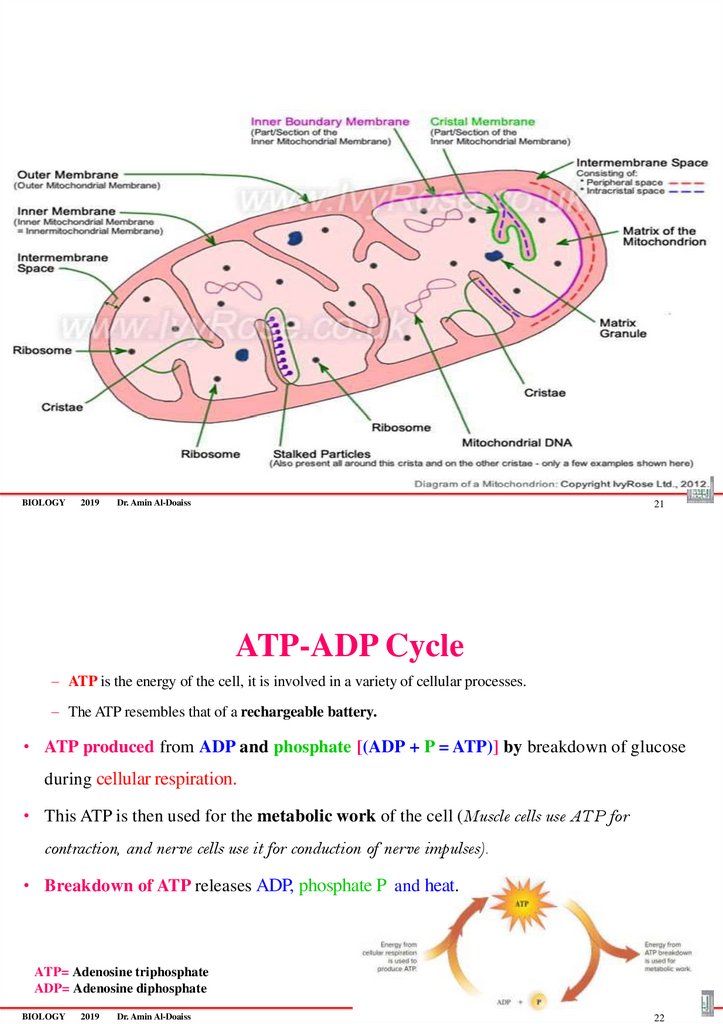
•Each mitochondrion possesses double
membrane:
1. Outer smooth membrane
2. Inner irregular folded membrane (Cristae)
–Between the membranes there is an intermembrane space
–Between the cristae there is a matrix space
BIOLOGY
2019
Dr. Amin Al-Doaiss
20
82.
BIOLOGY2019
Dr. Amin Al-Doaiss
21
ATP-ADP Cycle
– ATP is the energy of the cell, it is involved in a variety of cellular processes.
– The ATP resembles that of a rechargeable battery.
• ATP produced from ADP and phosphate [(ADP + P = ATP)] by breakdown of glucose
during cellular respiration.
• This ATP is then used for the metabolic work of the cell (Muscle cells use ATP for
contraction, and nerve cells use it for conduction of nerve impulses).
• Breakdown of ATP releases ADP, phosphate P and heat.
ATP= Adenosine triphosphate
ADP= Adenosine diphosphate
BIOLOGY
2019
Dr. Amin Al-Doaiss
22
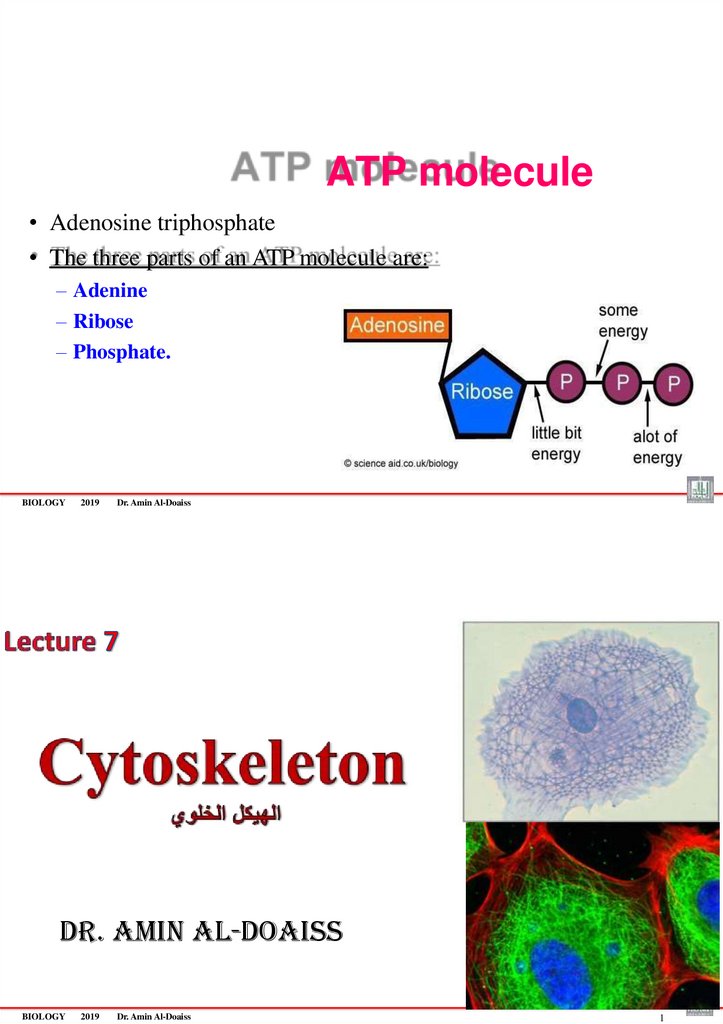
83. ATP molecule
• Adenosine triphosphate• The three parts of an ATP molecule are:
– Adenine
– Ribose
– Phosphate.
BIOLOGY
2019
Dr. Amin Al-Doaiss
Dr. Amin Al-Doaiss
BIOLOGY
2019
Dr. Amin Al-Doaiss
1
84.
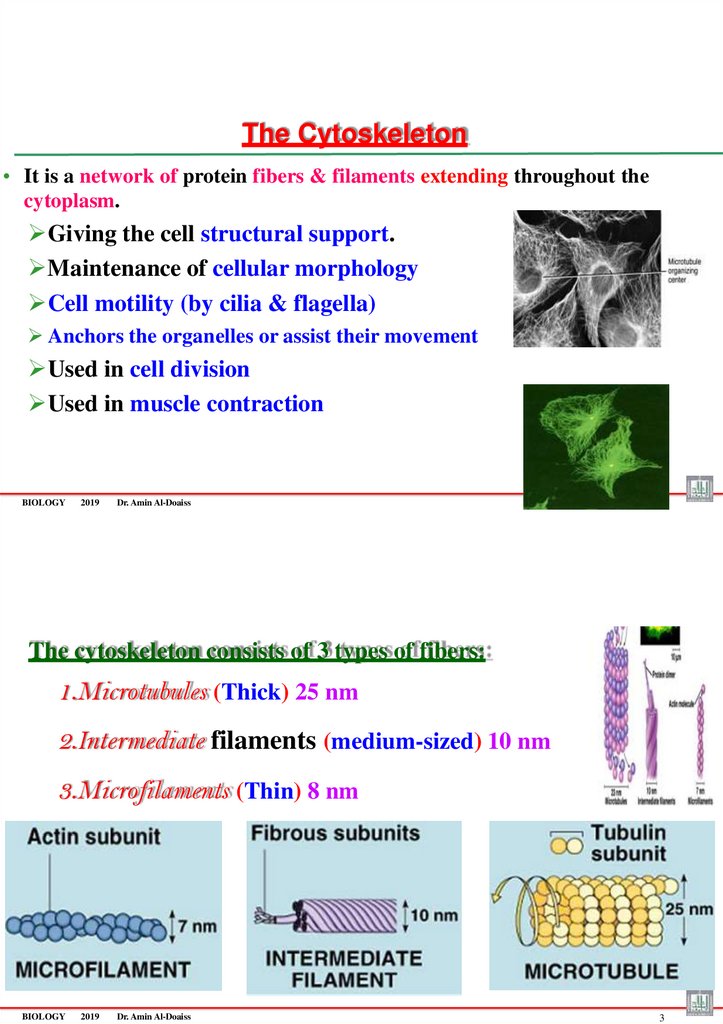
The Cytoskeleton• It is a network of protein fibers & filaments extending throughout the
cytoplasm.
Giving the cell structural support.
Maintenance of cellular morphology
Cell motility (by cilia & flagella)
Anchors the organelles or assist their movement
Used in cell division
Used in muscle contraction
BIOLOGY
2019
2
Dr. Amin Al-Doaiss
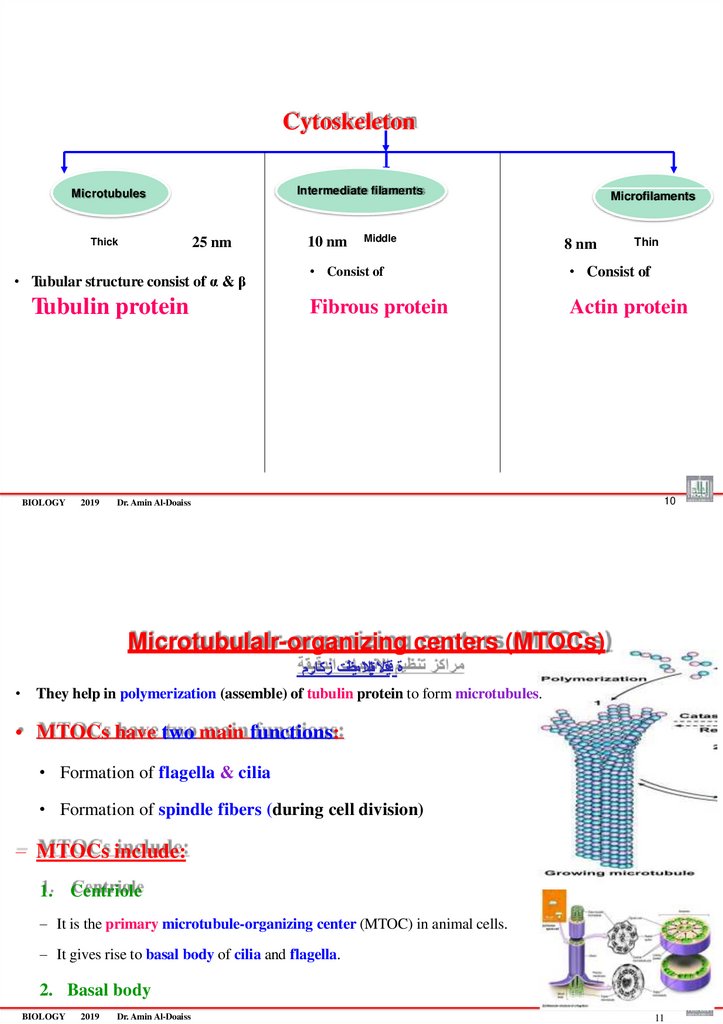
The cytoskeleton consists of 3 types of fibers:
1.Microtubules (Thick) 25 nm
2.Intermediate filaments (medium-sized) 10 nm
3.Microfilaments (Thin) 8 nm
BIOLOGY
2019
Dr. Amin Al-Doaiss
3
85.
BIOLOGY2019
Dr. Amin Al-Doaiss
4
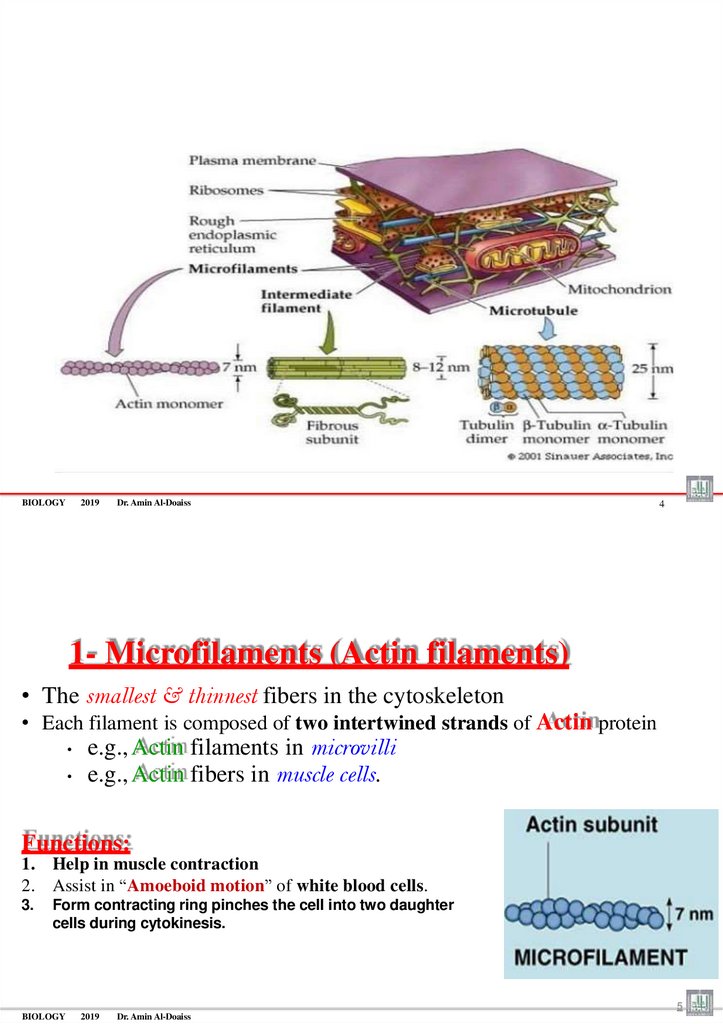
1- Microfilaments (Actin filaments)
• The smallest & thinnest fibers in the cytoskeleton
• Each filament is composed of two intertwined strands of Actin protein
e.g., Actin filaments in microvilli
e.g., Actin fibers in muscle cells.
Functions:
1. Help in muscle contraction
2. Assist in “Amoeboid motion” of white blood cells.
3.
Form contracting ring pinches the cell into two daughter
cells during cytokinesis.
5
BIOLOGY
2019
Dr. Amin Al-Doaiss
86.
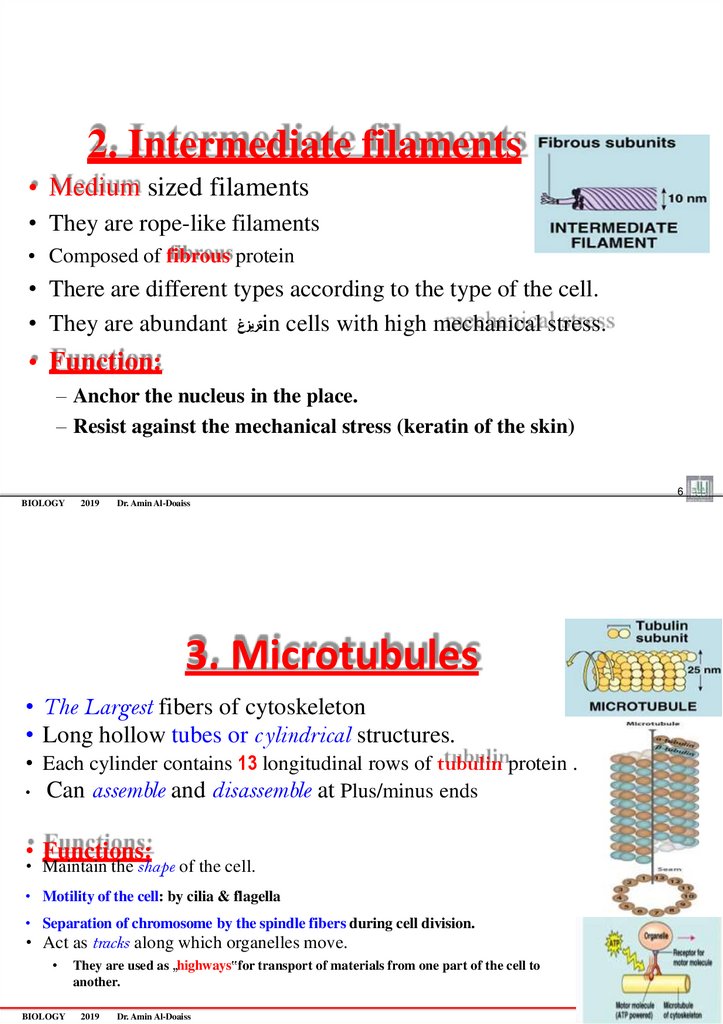
2. Intermediate filaments• Medium sized filaments
• They are rope-like filaments
• Composed of fibrous protein
• There are different types according to the type of the cell.
• They are abundant ةريزغin cells with high mechanical stress.
• Function:
– Anchor the nucleus in the place.
– Resist against the mechanical stress (keratin of the skin)
6
BIOLOGY
2019
Dr. Amin Al-Doaiss
3. Microtubules
• The Largest fibers of cytoskeleton
• Long hollow tubes or c ylindrical structures.
• Each cylinder contains 13 longitudinal rows of tubulin protein .
• Can assemble and disassemble at Plus/minus ends
• Functions:
• Maintain the shape of the cell.
• Motility of the cell: by cilia & flagella
• Separation of chromosome by the spindle fibers during cell division.
• Act as tracks along which organelles move.
BIOLOGY
They are used as „highways‟for transport of materials from one part of the cell to
another.
2019
Dr. Amin Al-Doaiss
7
87.
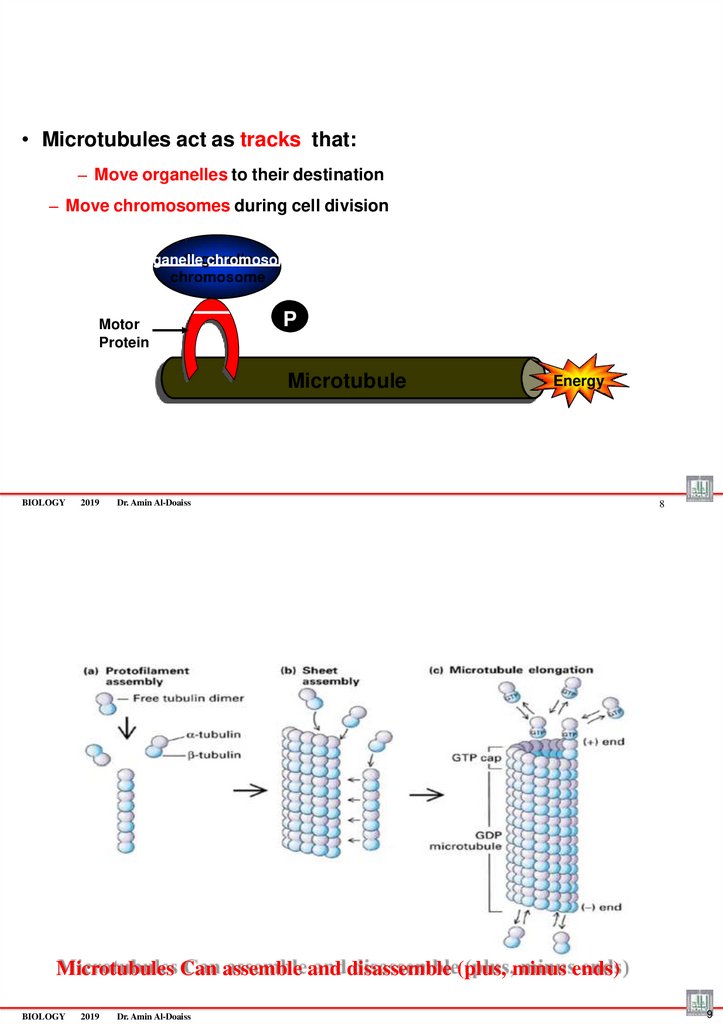
• Microtubules act as tracks that:– Move organelles to their destination
– Move chromosomes during cell division
Organelle chromosome
Motor
Protein
P
Microtubule
BIOLOGY
2019
Energy
Dr. Amin Al-Doaiss
8
Microtubules Can assemble and disassemble (plus, minus ends)
BIOLOGY
2019
Dr. Amin Al-Doaiss
9
88.
CytoskeletonIntermediate filaments
Microtubules
25 nm
Thick
• Tubular structure consist of α & β
Tubulin protein
BIOLOGY
2019
10 nm
Middle
Microfilaments
8 nm
Thin
• Consist of
• Consist of
Fibrous protein
Actin protein
10
Dr. Amin Al-Doaiss
Microtubulalr-organizing centers (MTOCs)
ة قيقدالتايبنالا ميظنت زكارم
• They help in polymerization (assemble) of tubulin protein to form microtubules.
• MTOCs have two main functions:
• Formation of flagella & cilia
• Formation of spindle fibers (during cell division)
– MTOCs include:
1. Centriole
– It is the primary microtubule-organizing center (MTOC) in animal cells.
– It gives rise to basal body of cilia and flagella.
2. Basal body
BIOLOGY
2019
Dr. Amin Al-Doaiss
11
89.
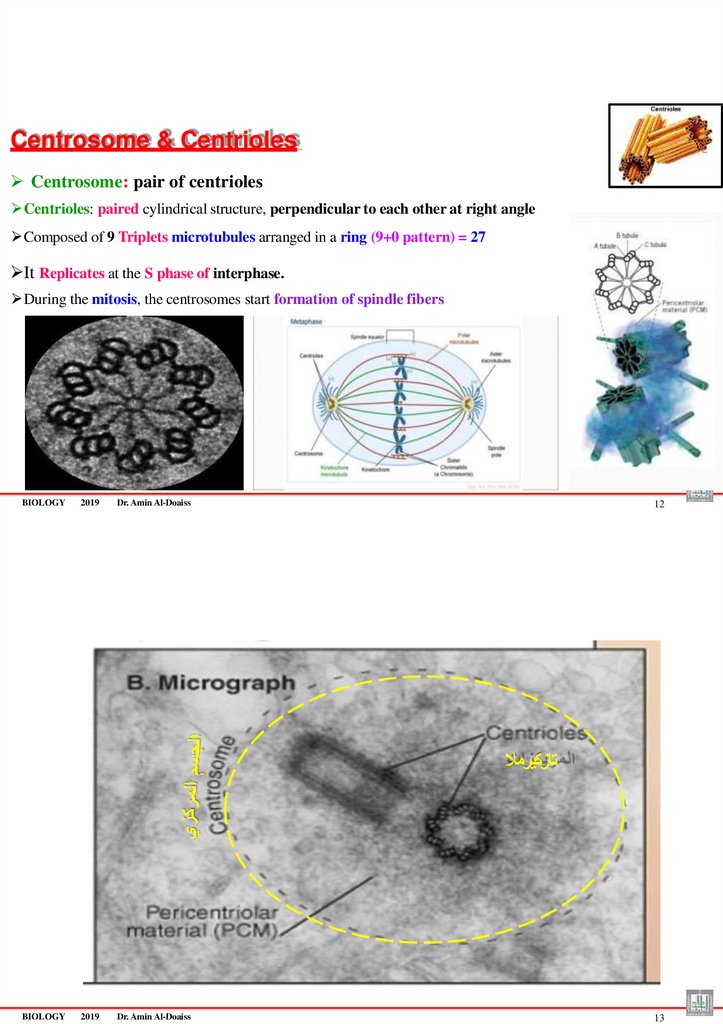
Centrosome & CentriolesCentrosome: pair of centrioles
Centrioles: paired cylindrical structure, perpendicular to each other at right angle
Composed of 9 Triplets microtubules arranged in a ring (9+0 pattern) = 27
It Replicates at the S phase of interphase.
During the mitosis, the centrosomes start formation of spindle fibers
BIOLOGY
2019
Dr. Amin Al-Doaiss
12
تازكيرمال
BIOLOGY
2019
Dr. Amin Al-Doaiss
13
90.
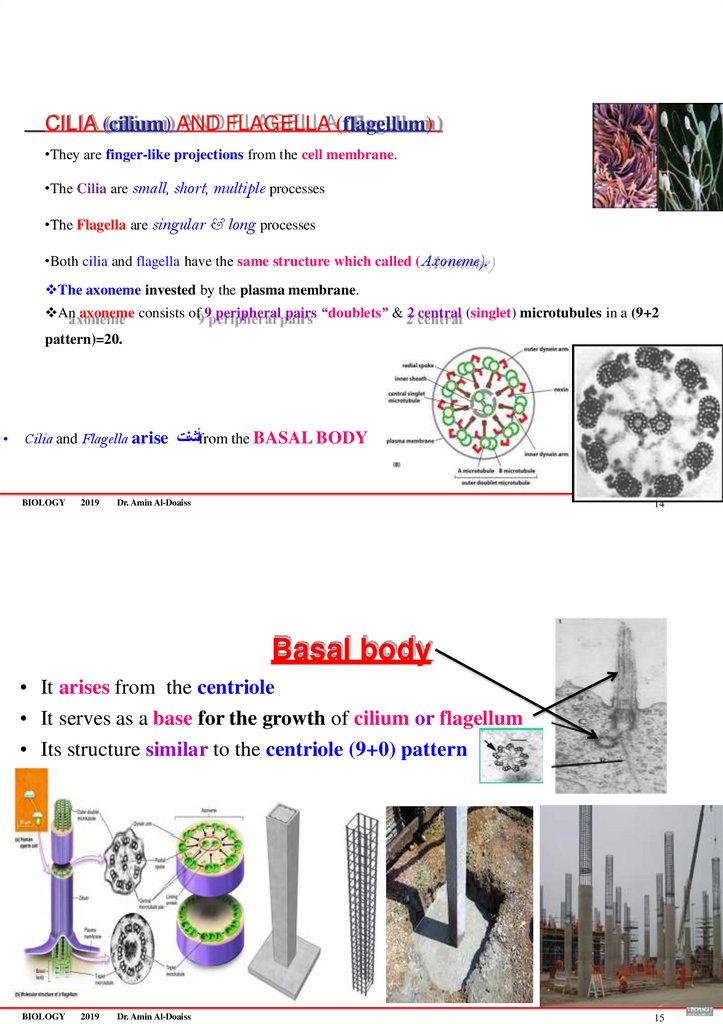
CILIA (cilium) AND FLAGELLA (flagellum)•They are finger-like projections from the cell membrane.
•The Cilia are small, short, multiple processes
•The Flagella are singular & long processes
•Both cilia and flagella have the same structure which called (Axoneme).
The axoneme invested by the plasma membrane.
An axoneme consists of 9 peripheral pairs “doublets” & 2 central (singlet) microtubules in a (9+2
pattern)=20.
• Cilia and Flagella arise أشنتfrom the BASAL BODY
BIOLOGY
2019
Dr. Amin Al-Doaiss
14
Basal body
• It arises from the centriole
• It serves as a base for the growth of cilium or flagellum
• Its structure similar to the centriole (9+0) pattern
BIOLOGY
2019
Dr. Amin Al-Doaiss
15
91.
Dr. Amin Al-DoaissBIOLOGY
2019
Dr. Amin Al-Doaiss
1
Cellular metabolism
• The sum of all of the chemical reactions occurring in a cell is called
metabolism
• Metabolism is all chemical reactions, which used to obtain
energy & growth.
• Types of metabolic reactions:
• Anabolism يئانب ضيا
• Catabolism يمده ضيا
BIOLOGY
2019
Dr. Amin Al-Doaiss
2
92.
Types of metabolic reactions :1. Anabolism: A metabolic reaction that leads to synthesis
of large molecules from smaller units.
• It is energy requiring
• Example: Photosynthesis, glycogenesis
2. Catabolism: A metabolic reaction that leads to breakdown
of large molecules into smaller units.
• to produce energy
• Example: Glycolysis
BIOLOGY
2019
Dr. Amin Al-Doaiss
3
Breakdown
Proteins to Amino Acids
Starch to Glucose
Synthesis
Amino Acids to Proteins
Glucose to Starch
BIOLOGY
2019
Dr. Amin Al-Doaiss
4
93.
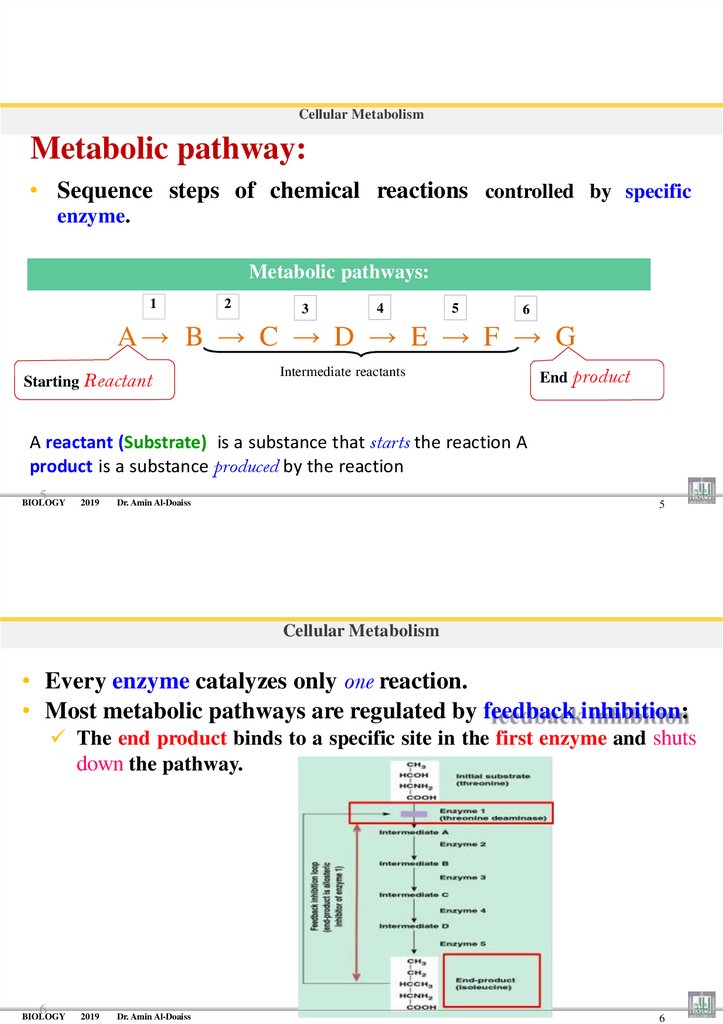
Cellular MetabolismMetabolic pathway:
• Sequence steps of chemical reactions controlled by specific
enzyme.
Metabolic pathways:
1
2
3
4
5
6
A→ B → C → D → E → F → G
Starting Reactant
Intermediate reactants
End product
A reactant (Substrate) is a substance that starts the reaction A
product is a substance produced by the reaction
5
BIOLOGY
2019
Dr. Amin Al-Doaiss
5
Cellular Metabolism
• Every enzyme catalyzes only one reaction.
• Most metabolic pathways are regulated by feedback inhibition:
The end product binds to a specific site in the first enzyme and shuts
down the pathway.
6
BIOLOGY
2019
Dr. Amin Al-Doaiss
6
94.
Cellular MetabolismEnzymes :
• Majority of Enzymes are proteins
• Enzyme’s Specificity is caused by the shape of active site.
• Enzymes are biological catalysts
• Catalyst: is a chemical that accelerate the chemical reaction
without being consumed.
• Enzymes are Recycled (reusable).
7
BIOLOGY
2019
Dr. Amin Al-Doaiss
7
Cellular Metabolism
Enzymes :
•Every reaction requires its specific enzyme; therefore, they are named for
substrates by adding the suffix “-ase.”
Enzymes Named for Their Substrates
8
Substrate
Enzyme
Lipid
Lipase
Urea
Maltose
Urease
Maltase
Ribonucleic acid
Ribonuclease
Lactose
Lactase
95.
Cellular MetabolismEnzyme-Substrate Complex
Enzymes form a complex with their substrates at the active site.
•Active site is small region on surface of enzyme where the substrate(s) bind.
•When substrate binds to enzyme, active site undergoes a slight change
in shape that facilitates the reaction, this is called the induced-fit
model, (Key & Lock Theory)
9
BIOLOGY
2019
Dr. Amin Al-Doaiss
Coenzymes and Co-factors
•Co-factors are helper substances that are
inorganic ions such as magnesium, zinc, or
manganese.
•Coenzymes are helper substances that are
organic molecules (e.g., vitamins).
•Co-factors or coenzymes bind to the active site and
change the shape of the active site so the substrate
now fits.
Vitamin deficiency causes lack of coenzyme and lack of enzymatic action.
10
9
96.
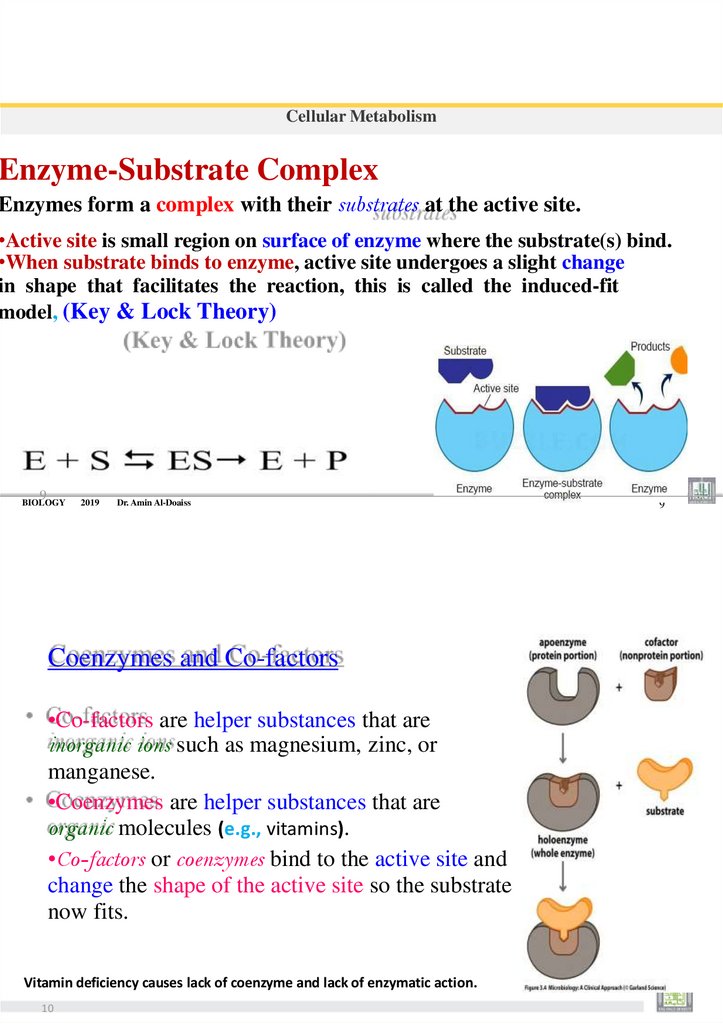
Cellular MetabolismCellular Respiration Aerobic respiration
• Cellular respiration converts the energy in the bonds of glucose into usable energy for the cell (ATP).
• There are Four Stages of Cellular Respiration
1.Glycolysis. 2.Preparatory reaction
3.Citric Acid (Krebs )Cycle.
4.Electron Transport System (chain)
1
11
Cellular Metabolism
12
2
3
4
97.
Cellular MetabolismGlycolysis the process of splitting a sugar.
• glycolysis is the first step in both aerobic and anaerobic respiration
Glycolysis can occur with or without O2
• Glycolysis Takes place in cytoplasm (cytosol)
• Glycolysis is a catabolic pathway,
• Glucose (C6) is split in half to 2 molecules of pyruvate or pyruvic acid (C3)
• There are 10 steps in glycolysis
• Used=2ATP, produces=4ATP, net=2ATP
• Results:
2 pyruvate
2 ATP
2 NADH
13
14
The ATP Input and Output of glycolysis
Cellular Metabolism
98.
Cellular MetabolismPreparatory (transition, link) reaction
Takes place in the matrix of the mitochondria.
Pyruvate (3C) produced by glycolysis is converted to acetyl CoA (2C).
A CO2 is removed from pyruvate – making a 2C compound.
Pyruvate loses one carbon atom to form acetyl CoA= Oxidation of pyruvate to acetyl CoA
Links between glycolysis and Krebs cycle
Results:
2 Acetyl CoA
2 CO2
NO ATP
pyruvate(C3)
→
acetylCoA(C2)
+ CO2
15
Cellular Metabolism
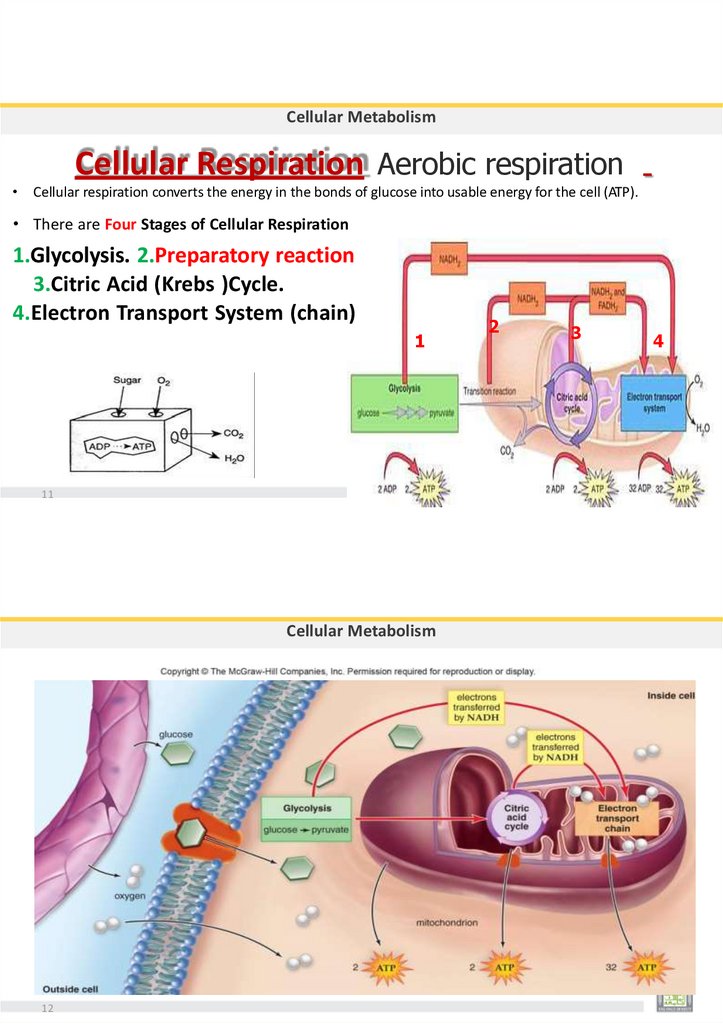
Krebs Cycle (Citric Acid Cycle)
•Takes place in the matrix of the mitochondria.
•Coenzyme A (2C) is attached to a oxaloacetic acid (4C) producing a citric
acid (6C).
•Occurs twice, once for each acetyl CoA.
•Results:
16
6
2
2
4
NADH
FADH2
ATP
CO2 Molecules.
99.
Cellular Metabolismcitric acid
Input
Output
Acetic acid
2 CO2
ADP
Krebs Cycle
3 NAD
FAD
oxaloacetic acid
17
Cellular Metabolism
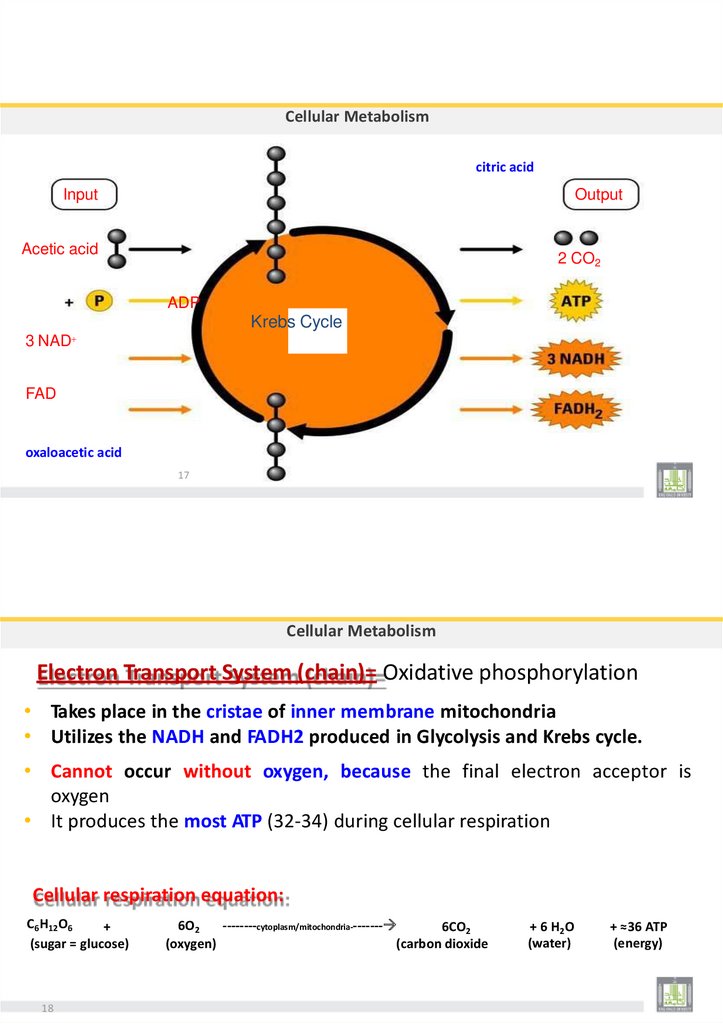
Electron Transport System (chain)= Oxidative phosphorylation
• Takes place in the cristae of inner membrane mitochondria
• Utilizes the NADH and FADH2 produced in Glycolysis and Krebs cycle.
• Cannot occur without oxygen, because the final electron acceptor is
oxygen
• It produces the most ATP (32-34) during cellular respiration
Cellular respiration equation:
C6H12O6
+
(sugar = glucose)
18
6O2
--------cytoplasm/mitochondria--------
6CO2
(oxygen)
(carbon dioxide
+ 6 H 2O
(water)
+ ≈36 ATP
(energy)
100.
Cellular Metabolism19
Cellular Metabolism
20




























 Биология
Биология








