Похожие презентации:
Chemistry of Life. Lecture 2
1.
Lecture 2.Chemistry of Life
2.
Matter• Living organisms, as every other thing, are
composed of matter, which is defined as
anything that takes up space and has mass.
• Matter is made up of elements.
3.
Elements• An element is a substance that
cannot be broken down to other
substances by chemical reactions.
• ~ 120 elements, 92 in nature.
• Each element consists of a certain
type of atom that is different from
the atoms of any other element.
• An atom is the smallest unit of
matter that still retains the
properties of an element.
• Substances which are made of
just one type of atom are called
pure.
.
4.
compoundsWater H2O
Na
Cl
NaCl
• A compound is a substance
consisting of two or more
different elements combined
in a fixed ratio.
• A compound has a unique
composition that is always the
same.
• The smallest particle of a
compound is called a
molecule.
5.
The Elements of Life• Of the 92 natural elements, about 20–25% are essential elements
that an organism needs to live. The essential elements are similar
among organisms, but there is some variation—for example,
humans need 25 elements, but plants need only 17.
• Four elements—oxygen (O), carbon (C), hydrogen (H), and nitrogen
(N)—make up 96% of living matter.
• Calcium (Ca), phosphorus (P), potassium (K), sulfur (S), and a few
other elements account for most of the remaining 4% of an
organism’s mass.
• Trace elements are required by an organism in only minute
quantities. Some trace elements, such as iron (Fe), are needed by
all forms of life; others are required only by certain species.
6.
Carbon: The Backbone of Life• Compounds found mainly in living things are known as organic
compounds.
• Organic compounds make up the cells and other structures of
organisms and carry out life processes.
• The main element in the organic compounds is carbon.
• Carbon is so basic to life thanks to its ability to form strong bonds
with many elements, including itself.
• This property allows carbon to form a huge variety of very large
complex molecules.
• However, the huge variety of organic compounds which form
living organisms can be grouped in just four major types:
carbohydrates, lipids, proteins and nucleic acids.
7.
Macromolecules• On the molecular scale, members of three of these classes—
carbohydrates, proteins, and nucleic acids—are huge and are
therefore called macromolecules.
• For example, a protein may consist of thousands of atoms.
• The macromolecules are chain-like structures called polymers (from
the Greek polys, many, and meros, part).
• A polymer is a long molecule consisting of many similar or identical
building blocks linked by covalent bonds, much as a train consists of
a chain of cars.
• The repeating units that serve as the building blocks of a polymer are
smaller molecules called monomers (from the Greek monos, single).
• Some of the molecules that serve as monomers also have other
functions of their own.
8.
carbohydrates: monosaccharides• Carbohydrates include both sugars and
polymers of sugars.
• The simplest carbohydrates are the
monosaccharaides, or simple sugars.
• Contain C-chains (> 3 atoms), H and O.
• These are the monomers from which
more complex carbohydrates are
constructed.
• Functions:
• major nutrients for cells
• raw material for the synthesis of
other types of small organic
molecules
9.
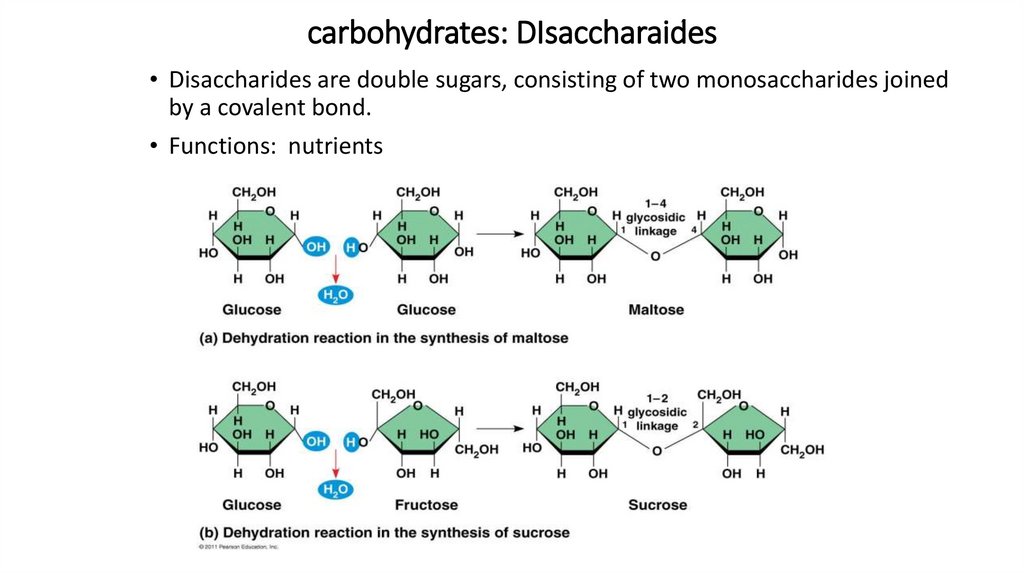
carbohydrates: DIsaccharaides• Disaccharides are double sugars, consisting of two monosaccharides joined
by a covalent bond.
• Functions: nutrients
10.
carbohydrates: Polysaccharides• Carbohydrates also include
macromolecules called
polysaccharides, polymers
composed of many sugar
building blocks.
• Functions:
• storage material (starch,
glycogen)
• building material for
structures that protect the
cell or the whole organism
(cellulose, chitine)
11.
Lipids mix poorly, if at all, with water12.
Lipids: FATS• A fat is constructed from two kinds of
smaller molecules: glycerol and fatty acids.
• Glycerol is an alcohol; each of its three
carbons bears a hydroxyl group.
• A fatty acid has a long carbon skeleton,
usually 16 or 18 carbon atoms in length.
The carbon at one end of the skeleton is
part of a carboxyl group, the functional
group that gives these molecules the
name fatty acid.
• The rest of the skeleton consists of a
hydrocarbon chain.
• The relatively nonpolar C¬H bonds in the
hydrocarbon chains of fatty acids are the
reason fats are hydrophobic.
13.
Saturated and unsaturated fats1. The major function of fats is energy storage (a gram of fat stores more than twice as much energy as a gram of a
polysaccharide, such as starch).
2. Body insulation (animals).
14.
Lipids: phospholipids• … are similar to fat molecules but
has only two fatty acids attached
to glycerol rather than three.
• The third hydroxyl group of
glycerol is joined to a phosphate
group, which has a negative
electrical charge in the cell.
• The hydrocarbon tails are
hydrophobic and the phosphate
group and its attachments form a
hydrophilic head that has an
affinity for water.
• … are building materials for cell
membranes.
15.
Lipids: Steroids• Steroids are lipids characterized by a carbon skeleton consisting of
four fused rings.
• E.g. cholesterol and the vertebrate sex hormones.
• Cholesterol is a common component of animal cell membranes and is
also the precursor from which other steroids are synthesized.
• In vertebrates, cholesterol is synthesized in the liver.
16.
Proteins• Proteins are large biological
molecules, or macromolecules,
consisting of one or more long
chains of amino acid residues.
• Amino acids are biologically
important organic compounds
composed of amine (-NH2) and
carboxylic
acid
(-COOH)
functional groups, along with a
side-chain specific to each.
• Polymers of amino acids are
called polypeptides.
17.
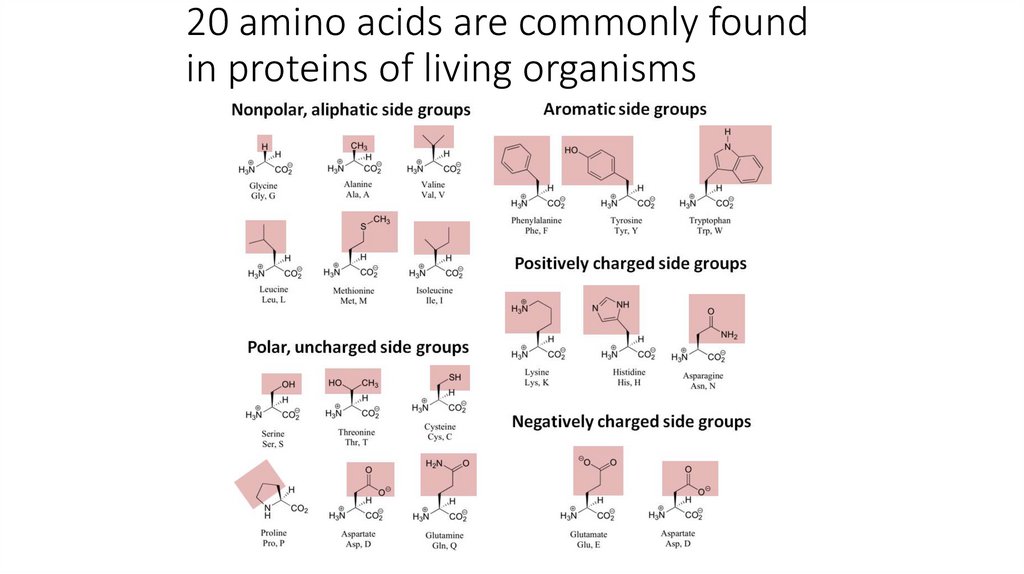
20 amino acids are commonly foundin proteins of living organisms
18.
Protein: Structure (1)• Two amino acids can be joined by
dehydration reaction (removal of
water molecule): -OH from the COOH groop and –H from amino
group.
• The resulting covalent bond is called
a peptide bond.
• Repeated over and over, this process
yields a polypeptide, a polymer of
many amino acids linked by peptide
bonds.
• The repeating sequence of atoms
highlighted in purple in is called the
polypeptide backbone.
19.
Protein: Structure (2)• All proteins share three superimposed levels of structure, known as
primary, secondary, and tertiary structure.
• A fourth level, quaternary structure, arises when a protein consists of
two or more polypeptide chains.
20.
Protein: Structure (3)Primary structure
The primary structure of a protein is a linked
series of amino acids with a unique sequence.
Secondary structure
The secondary structure is the result of hydrogen
bonds between the repeating components of the
polypeptide backbone (not the amino acid side chains)
21.
Protein: Structure (4)Tertiary structure
The tertiary structure is the overall shape of a
polypeptide resulting from interactions between
the side chains (R groups) of the various amino acids.
Quaternary structure
Some proteins consist of two or more polypeptide
chains aggregated into one functional macromolecule. Quaternary structure is the overall protein
structure that results from the aggregation of these
polypeptide subunits.
22.
Protein: function (1)23.
Protein: function (1)24.
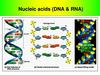
Nucleic acids (DNA & RNA)• Nucleic acids are macromolecules that
exist as polymers called polynucleotides.
• Each polynucleotide consists of
monomers called nucleotides.
• A nucleotide, in general, is composed
of three parts:
• a nitrogencontaining (nitrogenous) base,
• a five-carbon sugar (a pentose),
• and one or more phosphate groups.
(In a polynucleotide, each monomer has
only one phosphate group. The portion
of a nucleotide without any phosphate
groups is called a nucleoside.)
25.
Nitrogen containing (nitrogenous) bases• Each nitrogenous base has one or two
rings that include nitrogen atoms.
• There are two families of nitrogenous
bases: pyrimidines and purines.
• A pyrimidine has one six-membered
ring of carbon and nitrogen atoms
(cytosine (C), thymine (T), and uracil
(U)).
• Purines are larger, with a sixmembered ring fused to a fivemembered ring (adenine (A) and
guanine (G)).
26.
five-carbon sugars (pentoses)27.
how nucleotides are linked together tobuild a polynucleotide?
• Adjacent nucleotides are joined by a
phosphodiester linkage, which consists
of a phosphate group that links the
sugars of two nucleotides.
• This bonding results in a backbone with
a repeating pattern of sugar-phosphate
units (Note that the nitrogenous bases
are not part of the backbone).
• The sequence of bases along a DNA (or
mRNA) polymer is unique for each
gene and provides very specific
information to the cell.
28.
DNA and RNA structure• RNA molecules usually exist as single
polynucleotide chains.
• In contrast, DNA molecules have two
polynucleotides, or “strands,” that spiral around
an imaginary axis, forming a double helix.
• The sugarphosphate backbones are on the
outside of the helix, and the nitrogenous bases
are paired in the interior of the helix.
• The two strands are held together by hydrogen
bonds between the paired bases.
• Adenine (A) always pairs with thymine (T), and
guanine (G) always pairs with cytosine (C).
• Note that in RNA, adenine (A) pairs with uracil
(U); thymine (T) is not present in RNA.

























 Биология
Биология