Похожие презентации:
Review of the Vaccine Adverse Event Reporting System (VAERS)
1. Review of the Vaccine Adverse Event Reporting System (VAERS)
Beth Hibbs RN, MPH; Elaine Miller RN, MPHImmunization Safety Office (ISO)
Division of Healthcare Quality Promotion (DHQP)
Centers for Disease Control and Prevention
National Immunization Conference
April 21, 2010
1
2.
Educational Objectives• By the end of this presentation viewers
should be able to describe the following
about the Vaccine Adverse Event
Reporting System (VAERS):
– Role in vaccine safety surveillance
– Strengths and limitations
– Which adverse events should be reported to
VAERS
– How to report to VAERS
– How to perform a search of the VAERS data
• Identify resources for vaccine safety
2
3. Vaccine Adverse Event Definition
Adverse events are defined as healtheffects that occur after immunization
that may or may not be causally
related to the vaccine.
3
4. Vaccine Adverse Event Reporting System (VAERS)
• National spontaneous reporting system for adverseevents after US-licensed vaccines
– Received over 38,000 reports in 2009, (2005-2009
average per year ~29,000)
– Requires a report be filed; accepts reports from healthcare
providers, manufacturers and others
• Jointly administered CDC and FDA, Authorized by
National Childhood Vaccine Injury Act of 1986
– First reports accepted in 1990
• VAERS data publicly available on VAERS web site
or CDC Wide-ranging Online Data for
Epidemiologic Research (WONDER)
4
5. Purpose of VAERS
VAERS is used to:• Identify new and/or rare adverse events
following immunization
• Monitor trends of known adverse events
• Identify potential patient risk factors for
particular types of adverse events
• Generate hypotheses
• Provide information for public health policies
on vaccine safety
• Monitor vaccine lot safety
5
6. VAERS Strengths
• Can detect very rare adverseevents that may not be detected
before licensure
• Generates hypotheses
– Helps identify new and/or rare
adverse events following
immunization
– Helps determine if further
investigations are needed
• Monitors trends of already known
adverse events
• Monitors vaccine lot safety
6
7. VAERS Limitations
• Underreporting• Stimulated reporting due to
media attention and other factors
• Possibly incomplete and
inaccurate data on report form
• Lack of availability of
denominator data
– No information on number of
persons vaccinated
– No information on background rates
of adverse events in the population
• VAERS generally cannot determine if
an adverse event report was
coincidental or caused by a vaccine
7
8. VAERS Uses (Examples)
– General Safety of Vaccines• H1N1 influenza vaccines
Safety of Influenza A (H1N1) 2009 Monovalent Vaccines-US., Oct .1Nov. 24, 2009. MMWR 2009 Dec 11;58(48): 1351-1356.
– New signal, rare adverse events
• Intussusception after rotashield vaccine
Withdrawal of Rotavirus Vaccine Recommendation. MMWR 1999 Nov
5; 48(43): 1007.
• Myopericarditis after smallpox vaccine
Update: Cardiac and Other Adverse Events Following Civilian Smallpox
Vaccination --- United States, 2003. MMWR 2003 July 11; 52 (27):
639-642.
• Syncope following Vaccination
Syncope After Vaccination --- United States, January 2005--July 2007. MMWR
2008 May 2; 57(17): 457-60.
8
9. VAERS Uses (Examples) continued
– Reassuring Evidence SupportingVaccine Safety
• Guillain-Barre Syndrome risk identified
following 1976 influenza vaccine since then
VAERS has not identified a clear increase
in risk Prevention and Control of seasonal Influenza with vaccinesRecommendations of the ACIP, MMWR 2009 July 31;58(RR08): 1-52.
• Decreased risk of fever and seizures after
acellular compared to whole cell pertussis
vaccines Infant Immunization with Acellular Pertussis Vaccines in
the US: Assessment of the First Two Years’ Data from the Vaccine
Adverse Event Reporting System (VAERS). Braun et al. Pediatrics
2000 Oct;106(4): E51.
9
10. What to Report to VAERS
• Report any clinically significantadverse event following immunization
(www.vaers.hhs.gov)
– Even if you are not certain the vaccine
caused the event
• The National Childhood Vaccine
Injury Act of 1986 mandates that
healthcare providers also report
specific adverse events that occur
after vaccination
– Events listed in the Table of Reportable Events
https://kids.phila.gov/Docs/VAERS_Reportable
EventsTable.pdf
– Events listed in the vaccine package insert as
a contraindication to further doses of vaccine
10
11. What to Report to VAERS (continued)
• The report asks for informationabout pt, provider and reporter
demographics, adverse event ,
vaccines received and any
preexisting conditions.
demographics
AE
• Include as much information as
possible in the report (e.g.,
vaccination location, date, vaccine
type, lot number and dose number)
– Reports with incomplete
information accepted
• Report as soon as possible but no
time limit on reporting
11
vax
12. How to Submit a VAERS Report: One of Several Methods May Be Used
1) Online via a secure website athttps://vaers.hhs.gov
2) Download a reporting form:
http://vaers.hhs.gov/resources/vaers_for
m.pdf
• Fax a completed form: 877-721-0366
• Mail a completed VAERS form to VAERS,
P.O. Box 1100, Rockville, MD, 20849
To request a reporting form or for other VAERS
assistance: call 800-822-7967 or email:
info@vaers.org
12
13. VAERS Follow-up
• VAERS staff follow up withhealth care providers on serious
reports and certain selected
reports of interest by phone to
obtain:
– Medical records
– Autopsy reports
• Letter sent to reporters to check
recovery status for all reports
with “no” or “unknown” recovery
listed on initial VAERS form (60
days and 1 year)
13
14. How Does VAERS Define a “Serious” Report?*
DeathLife-threatening illness
Hospitalization
Prolongation of hospitalization
Persistent or significant
disability
Certain other medically
important conditions
*Code of Federal Regulations
Title 21
14
Box 8 of VAERS form
15. Selected Questions and Answers about VAERS
1516. How are VAERS Reports Analyzed? CDC and FDA have primary responsibility for analysis
• Assess for signals for new or unexpected adverse eventsof concern
– Use Medical Dictionary for Regulatory Activities (MedDRA) terms to
analyze frequencies of reported adverse events (focus on serious
adverse events)
– Doses distributed data are commonly used as a proxy for doses
administered
– Assess and compare reporting rates among vaccines and health
events of interest (observed in VAERS versus expected in general
population)
• Review individual reports for serious adverse events and
selected other conditions
– Additional information available from follow-up medical records and
autopsy reports
• Closely monitor safety of vaccine lots (FDA lead)
16
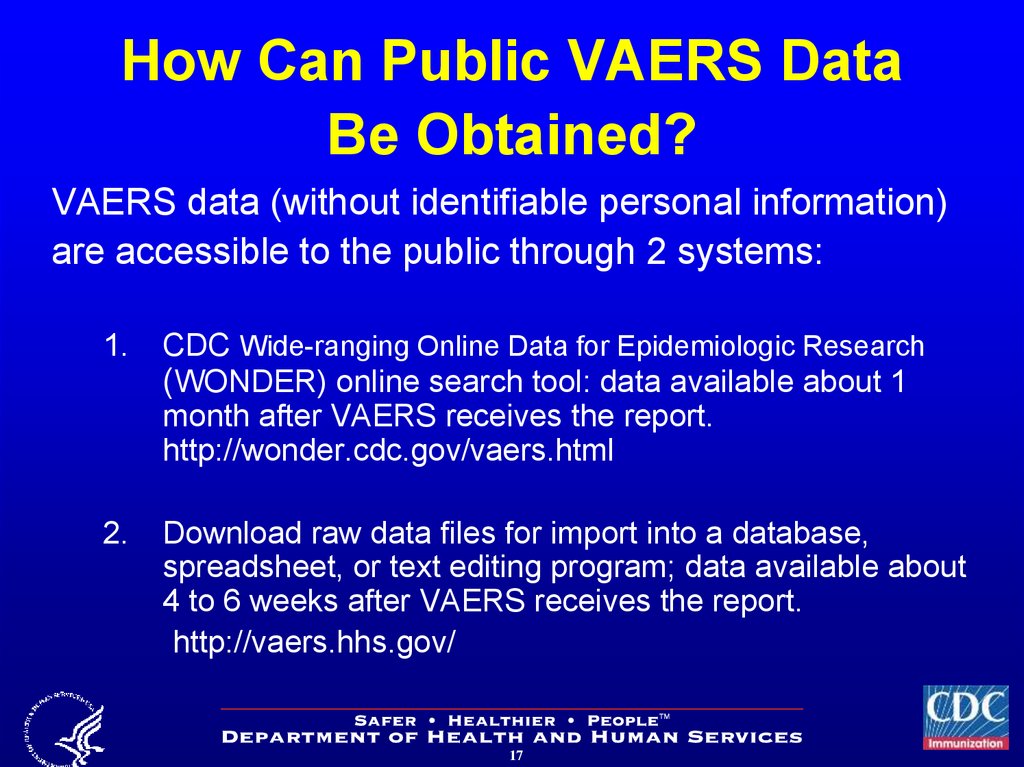
17. How Can Public VAERS Data Be Obtained?
VAERS data (without identifiable personal information)are accessible to the public through 2 systems:
1.
CDC Wide-ranging Online Data for Epidemiologic Research
(WONDER) online search tool: data available about 1
month after VAERS receives the report.
http://wonder.cdc.gov/vaers.html
2.
Download raw data files for import into a database,
spreadsheet, or text editing program; data available about
4 to 6 weeks after VAERS receives the report.
http://vaers.hhs.gov/
17
18. What Are the Best Resources for Vaccine Safety?
Publications updated with vaccine safetyresearch findings and recommendations:
• Manufacturer Vaccine Package Insert
– http://www.fda.gov/BiologicsBloodVaccines/Vaccines/Approv
edProducts/UCM093833
• Advisory Committee on Immunization
Practices Statements
– http://www.cdc.gov/vaccines/pubs/ACIP-list.htm
• Vaccine Information Statements
– http://www.cdc.gov/vaccines/pubs/vis/default.htm
18
19. How Can Healthcare and Vaccine Providers Contribute to Vaccine Safety?
• Properly store and administervaccine
http://www.cdc.gov/vaccines/recs/storage/default.htm
• Screen for contraindications and
precautions
http://www.cdc.gov/vaccines/recs/vacadmin/contraindications.htm
• Educate vaccinee (or caregiver)
about risks and benefits of vaccine
• Evaluate and treat patient if an
adverse event occurs
• Report clinically significant adverse
events promptly to VAERS
– https://vaers.hhs.gov
19
20. Continued: How Can Healthcare and Vaccine Providers Contribute to Vaccine Safety?
Patient Education Materials• CDC Vaccine Information
Statements (VIS)
http://www.cdc.gov/vaccines/Pubs/vis/
default.htm
Contains Vaccine Safety
information
– Contraindications
– VAERS
– Vaccine Injury Compensation
• Public health law requires
VIS to be distributed to
recipients of recommended
childhood vaccines
20
Example MMR VIS
21. HHS Vaccine Safety Resources
CDC
–
–
–
VAERS -CDC/FDA
–
–
800-822-7967
http://vaers.hhs.gov
Vaccine Injury Compensation- HRSA
–
–
Immunization Safety Office Web site
www.cdc.gov/vaccinesafety
800-CDC-INFO (232-4636)
CDCinfo@cdc.gov
800-338-2382
www.hrsa.gov/vaccinecompensation/
Food and Drug Administration
–
www.fda.gov/cber
21
22. VAERS Selected Bibliography
• Varricchio F, Iskander J, Destefano F, Ball R, Pless R, BraunMM, Chen RT. Understanding Vaccine Safety Information from
the Vaccine Adverse Event Reporting System. Pediatric
Infectious Disease Journal 2004;23(4):287-294.
• Iskander JK, Miller ER, Chen RT. The Role of the Vaccine
Adverse Event Reporting System (VAERS) in Monitoring
Vaccine Safety. Pediatr Ann. 2004 Sep;33(9):599-606.
• Iskander J, Pool V, Zhou W, English-Bullard R; The VAERS
Team. Data Mining in the US using the Vaccine Adverse Event
Reporting System. Drug Safety 2006;29(5):375–384.
Zhou W, Pool V, Iskander JK, English-Bullard R, et al. Surveillance for
safety after immunization: Vaccine Adverse Event Reporting System
(VAERS)—United States, 1991–2001. MMWR 2003;52(1):1–24.
22
23. Summary
• VAERS is a front-line mechanism to monitor thesafety of US Licensed vaccines
• The first hint of a potential problem usually originates
with the astute clinician and therefore the role of the
health professional is essential in identifying vaccine
adverse events through reports to VAERS
• VAERS report data are used to:
– inform CDC and FDA and others in vaccine safety
surveillance and research,
– identify possible rare or new vaccine side effects or changes
in known vaccine side effects,
– monitor lot safety,
– update the manufacturer package insert, Advisory
Committee on Immunization Practices (ACIP) vaccine
recommendations and Vaccine Information Statements.
23
24. Questions
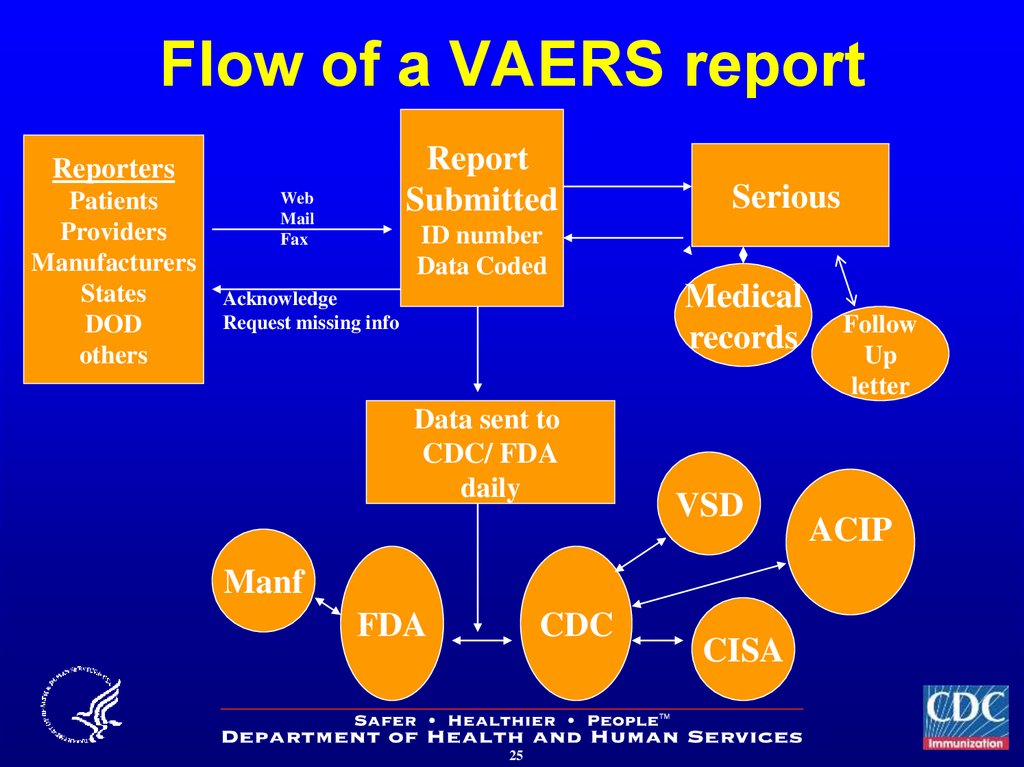
2425. Flow of a VAERS report
ReportSubmitted
Reporters
Patients
Providers
Manufacturers
States
DOD
others
Web
Fax
Serious
ID number
Data Coded
Medical
records
Acknowledge
Request missing info
Data sent to
CDC/ FDA
daily
VSD
Manf
FDA
CDC
25
CISA
Follow
Up
letter
ACIP
26. VAERS Background
• US post licensure vaccine safety surveillance– Collects voluntary reports of adverse events
following immunization
– Co-managed by CDC and the Food and Drug
Administration (FDA)
• Healthcare providers are encouraged to
report clinically significant adverse events
after vaccination*
– Anyone can submit a report to VAERS
• Receives ~23,000 reports per year (20052009 average)
• Data publicly available
*Clinically significant means of concern to the healthcare provider or
vaccinee/ care giver or other VAERS reporter; www.vaers.hhs.gov
26
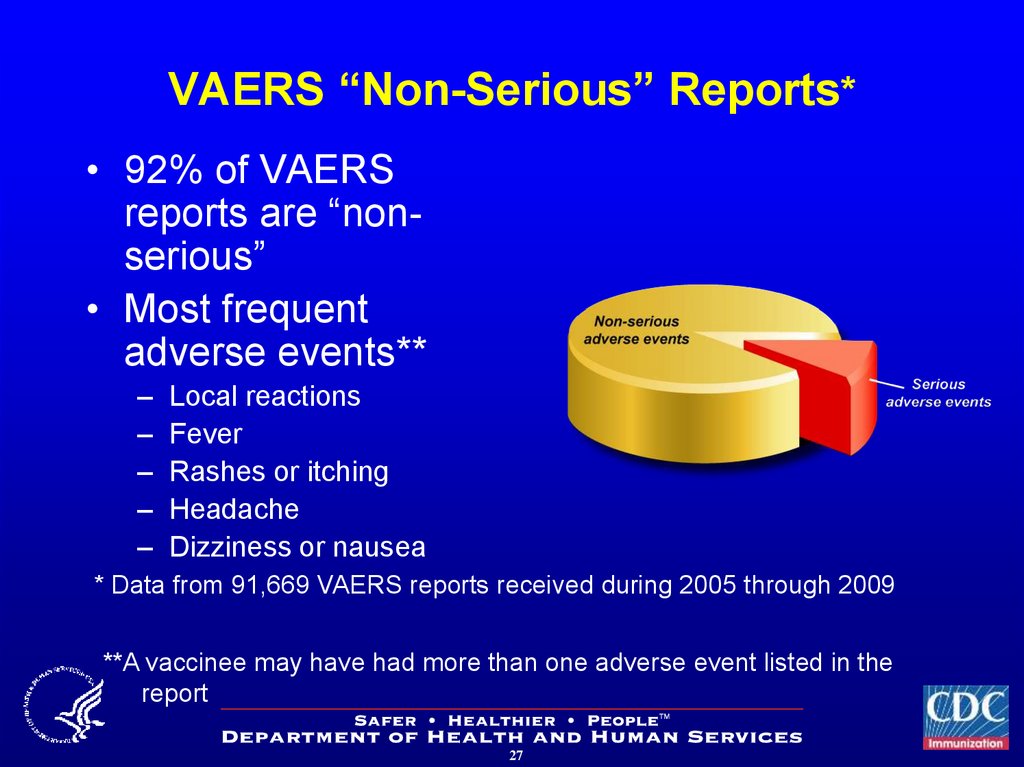
27. VAERS “Non-Serious” Reports*
• 92% of VAERSreports are “nonserious”
• Most frequent
adverse events**
–
–
–
–
–
Local reactions
Fever
Rashes or itching
Headache
Dizziness or nausea
* Data from 91,669 VAERS reports received during 2005 through 2009
**A vaccinee may have had more than one adverse event listed in the
report
27
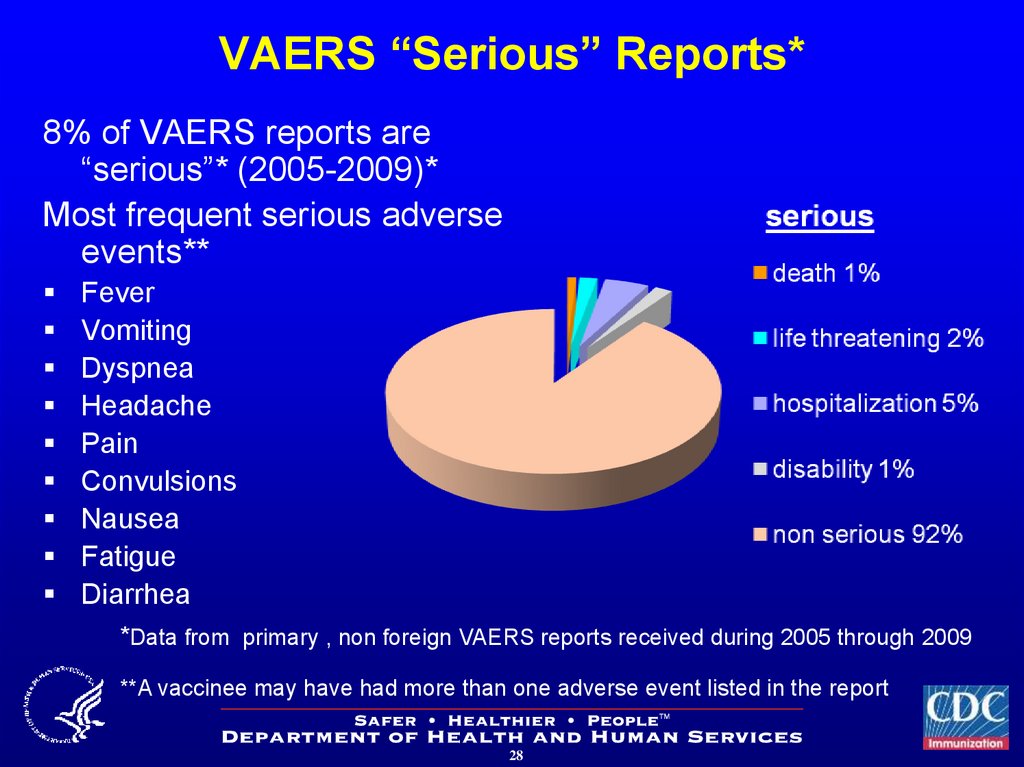
28. VAERS “Serious” Reports*
8% of VAERS reports are“serious”* (2005-2009)*
Most frequent serious adverse
events**
Fever
Vomiting
Dyspnea
Headache
Pain
Convulsions
Nausea
Fatigue
Diarrhea
*Data from primary , non foreign VAERS reports received during 2005 through 2009
**A vaccinee may have had more than one adverse event listed in the report
28






















 Медицина
Медицина








