Похожие презентации:
Carbohydrates
1.
Carbohydratesد .جهان عبد الوهاب
2.
BIOMEDICAL IMPORTANCECarbohydrates are widely distributed in plants and animals;
they have important structural and metabolic roles.
In plants, glucose is synthesized from carbon dioxide and
water by photosynthesis and stored as starch or used to
synthesize the cellulose of the plant cell walls.
Animals can synthesize carbohydrates from amino acids, but
most are derived ultimately from plants.
3.
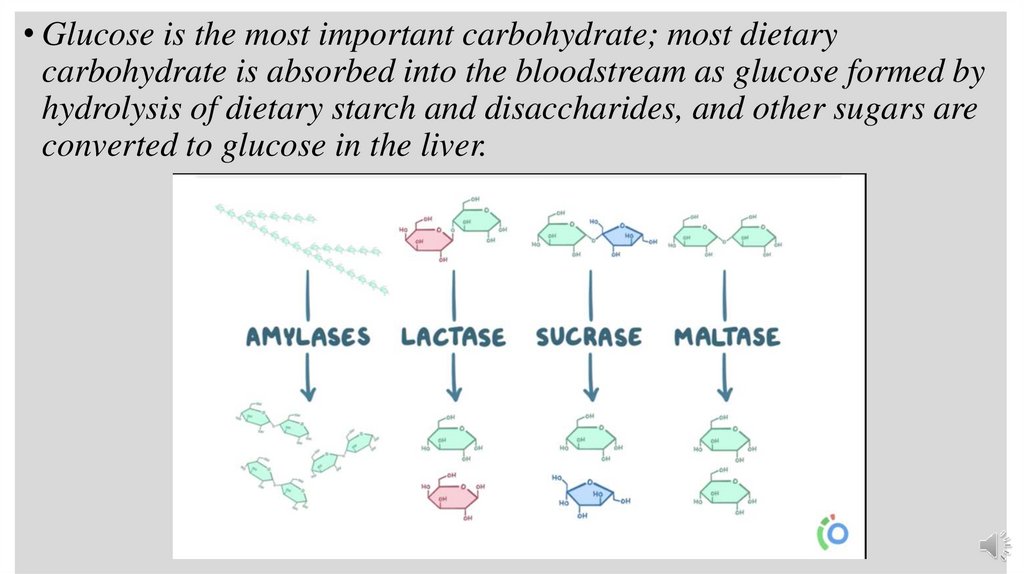
• Glucose is the most important carbohydrate; most dietarycarbohydrate is absorbed into the bloodstream as glucose formed by
hydrolysis of dietary starch and disaccharides, and other sugars are
converted to glucose in the liver.
4.
• Function of glucoseGlucose is the major metabolic fuel of mammals (except
ruminants).
a universal fuel of the fetus.
It is the precursor for synthesis of all the other carbohydrates in
the body, including glycogen for storage; ribose and deoxyribose
in nucleic acids; galactose in lactose of milk, in glycolipids, and in
combination with protein in glycoproteins and proteoglycans.
5.
Diseases associated with CHOdiabetes mellitus
Galactosemia
glycogen storage diseases
Lactose intolerance
6.
Carbohydrates (CHO)also called as saccharides, are most abundant biological
molecules containing C, H and O, which are combined as
(CH2O)n, where n is 3 or more. These may be defined as
aldehyde or ketone derivatives of polyhydric alcohols.
7.
Classification of CarbohydratesDepending upon the number of monomeric units present in a molecule,
carbohydrates are classified as :
1- Monosaccharides :
are those sugars that cannot be hydrolyzed into simpler carbohydrates.
They may be classified as trioses, tetroses, pentoses , hexoses, or heptoses,
depending upon the number of carbon atoms, and as aldoses or ketoses,
depending upon whether they have an aldehyde or ketone group.
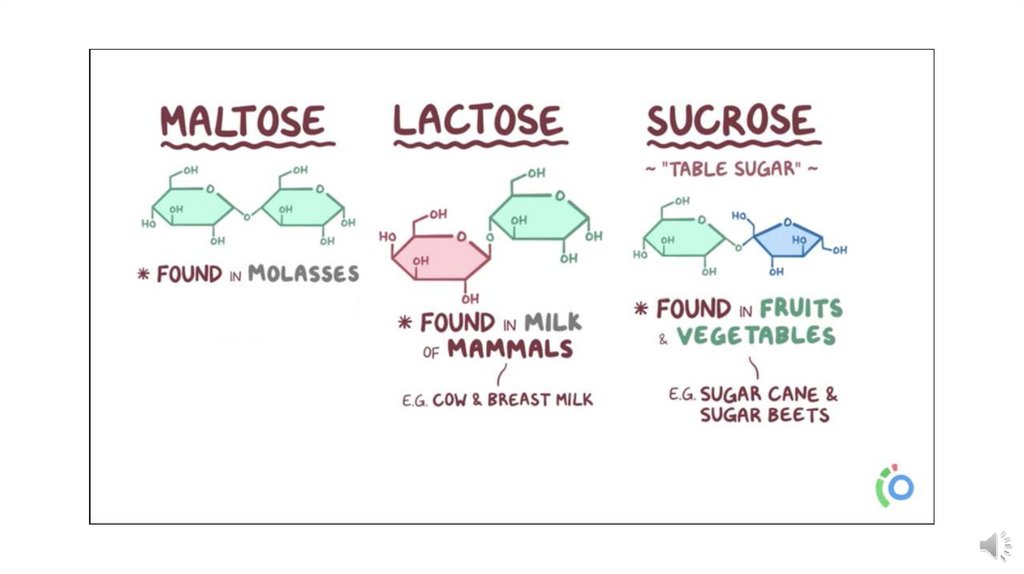
2- Disaccharides are condensation products of two monosaccharide units;
examples are maltose, lactose and sucrose.
8.
9.
10.
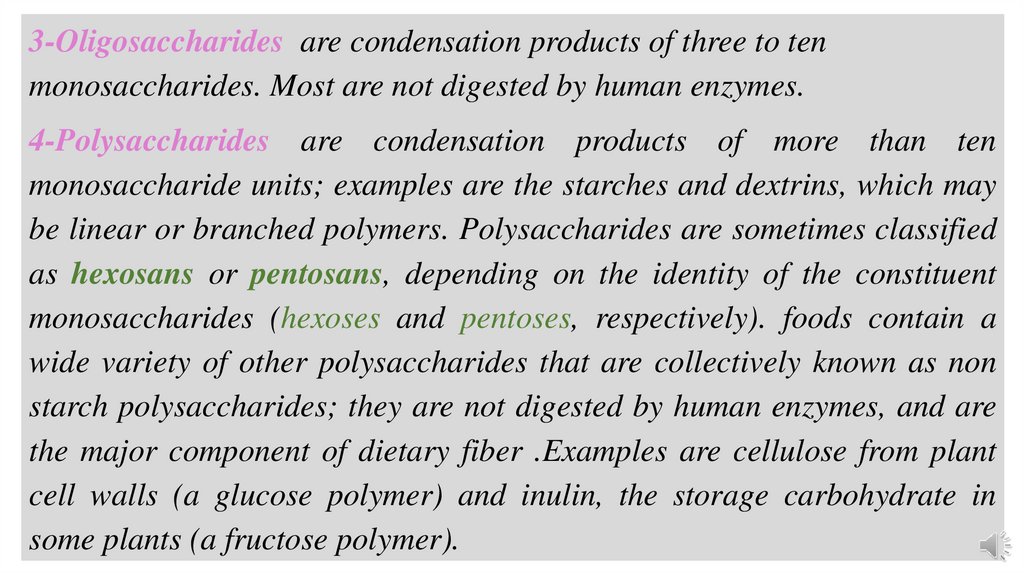
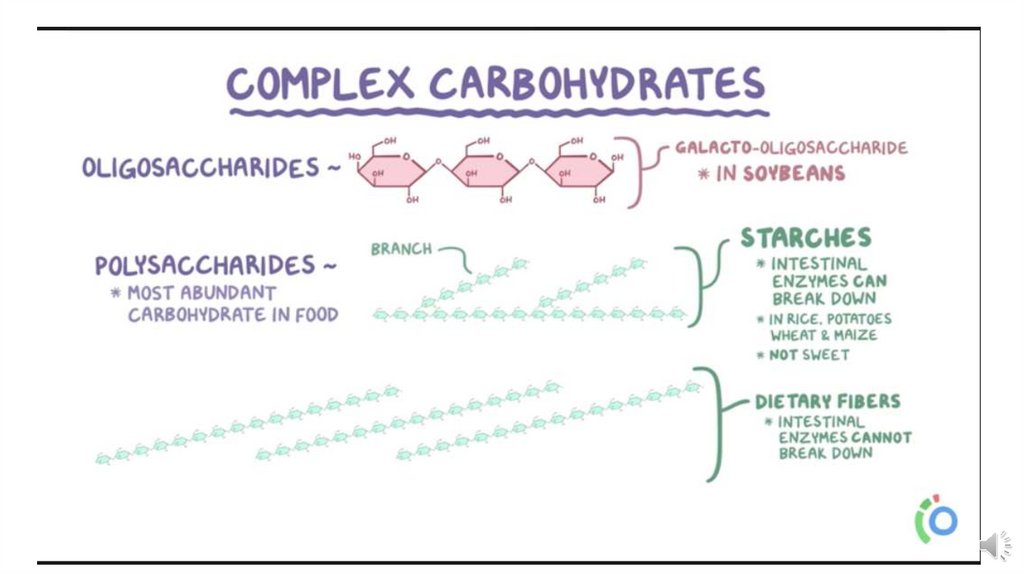
3-Oligosaccharides are condensation products of three to tenmonosaccharides. Most are not digested by human enzymes.
4-Polysaccharides are condensation products of more than ten
monosaccharide units; examples are the starches and dextrins, which may
be linear or branched polymers. Polysaccharides are sometimes classified
as hexosans or pentosans, depending on the identity of the constituent
monosaccharides (hexoses and pentoses, respectively). foods contain a
wide variety of other polysaccharides that are collectively known as non
starch polysaccharides; they are not digested by human enzymes, and are
the major component of dietary fiber .Examples are cellulose from plant
cell walls (a glucose polymer) and inulin, the storage carbohydrate in
some plants (a fructose polymer).
11.
12.
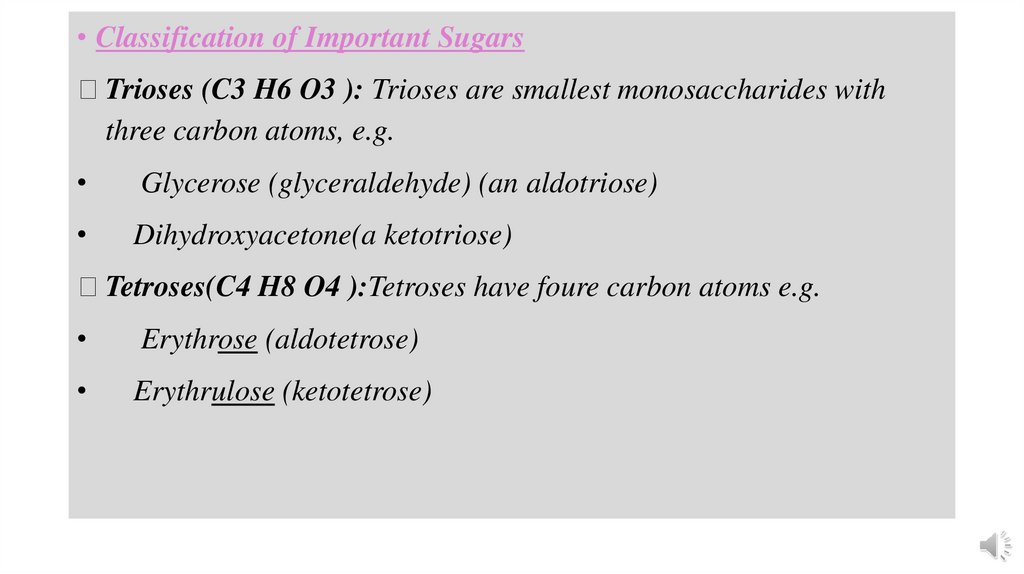
• Classification of Important Sugars Trioses (C3 H6 O3 ): Trioses are smallest monosaccharides with
three carbon atoms, e.g.
Glycerose (glyceraldehyde) (an aldotriose)
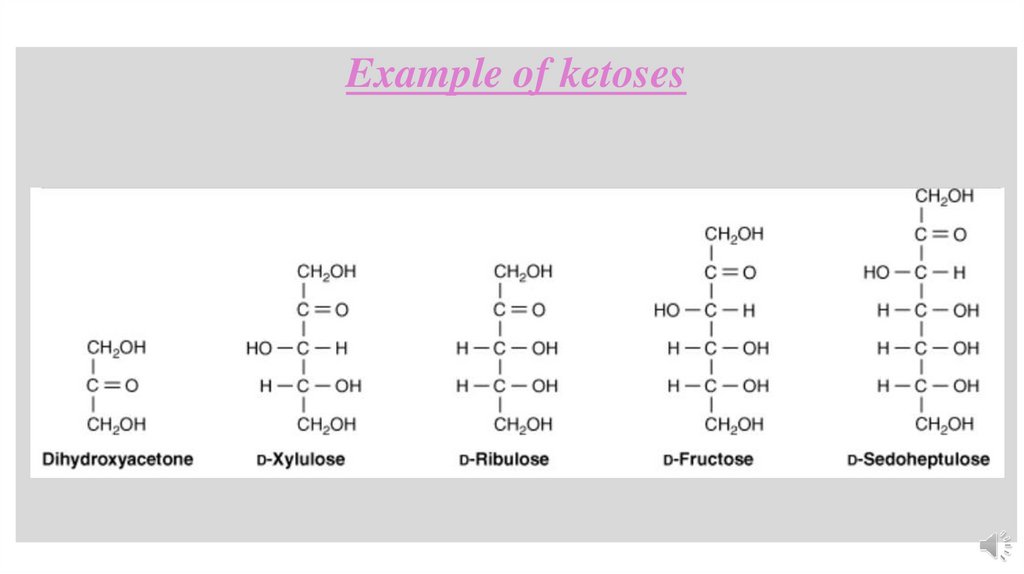
Dihydroxyacetone(a ketotriose)
Tetroses(C4 H8 O4 ):Tetroses have foure carbon atoms e.g.
Erythrose (aldotetrose)
Erythrulose (ketotetrose)
13.
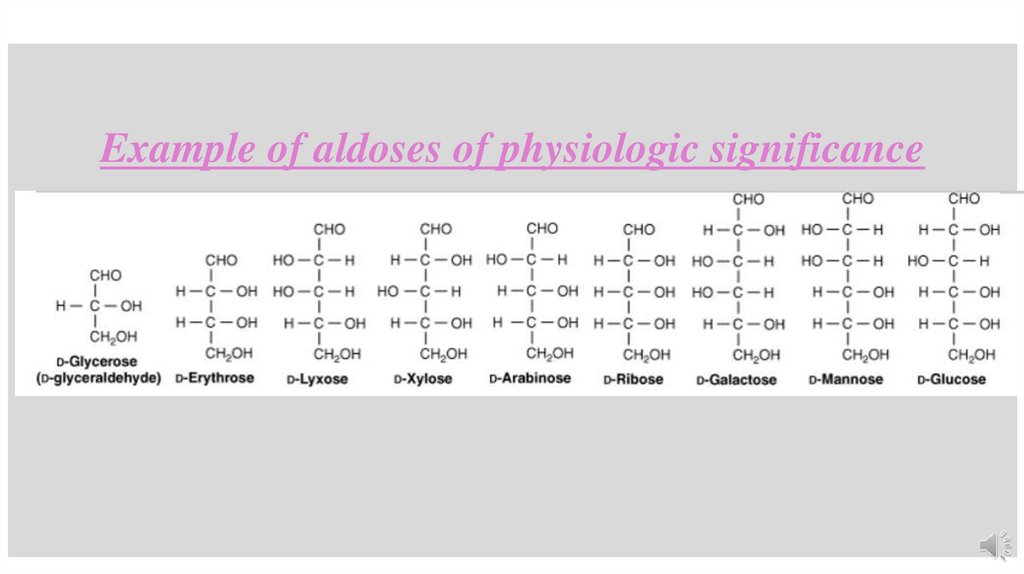
Pentoses (C5 H10 O5 ):Pentoses are sugars containing five carbon atoms, suchas
Ribose (an aldopentose)
Ribulose (a ketopentose),both of these are intermediates of the HMP
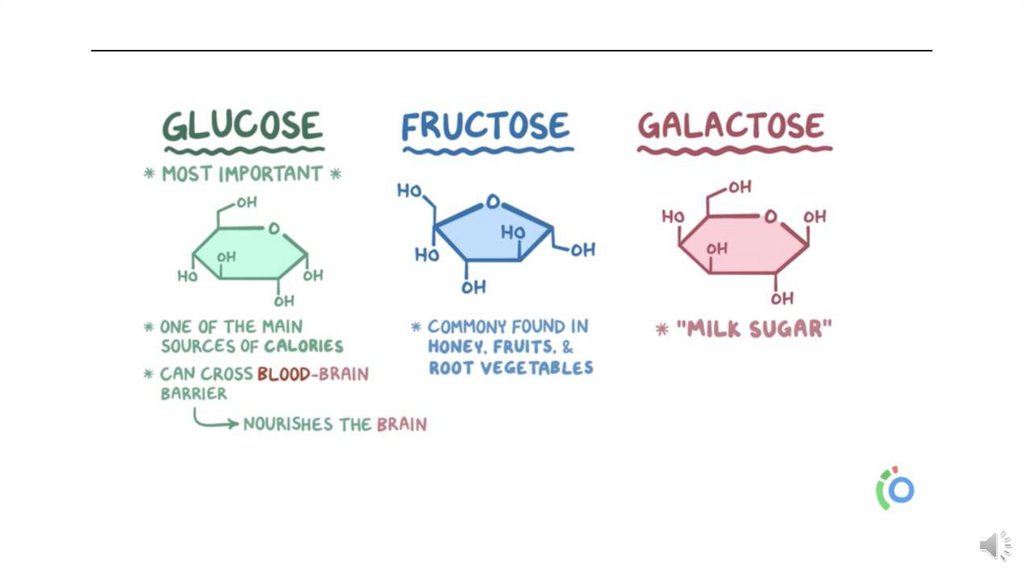
Hexoses (C6 H12 O6 ):Hexoses contain six carbon atoms, e.g.
• Glucose, commonly known as dextrose, is used as source of energy in the body.
• Fructose, is constituent of the common sugar, sucrose.
• Galactose, is a constituent of milk sugar lactose.
Heptoses (C7 H14 O7 )
—
Sedoheptulose
14.
Example of aldoses of physiologic significance15.
Example of ketoses16.
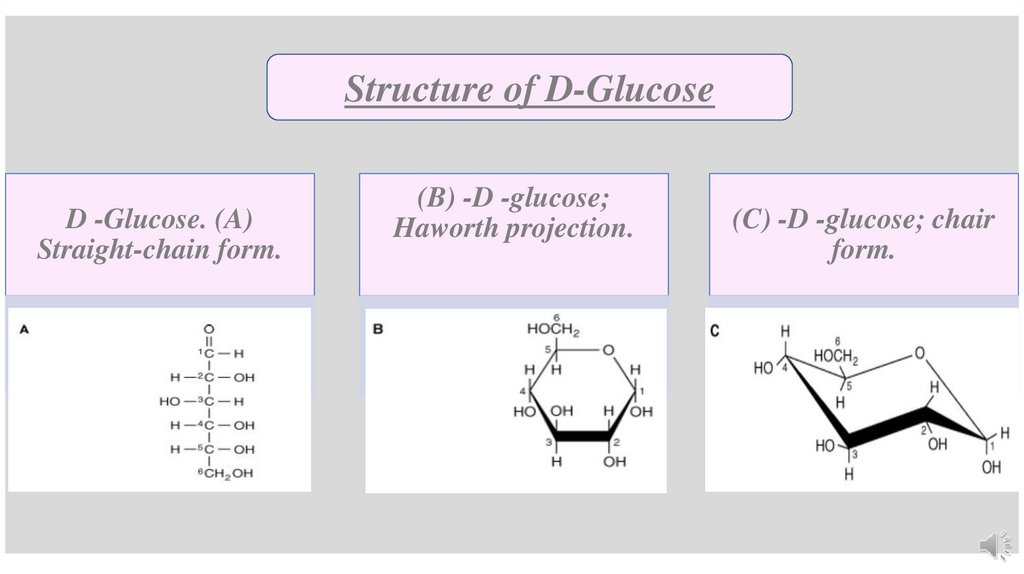
Structure of GlucoseThe structure of glucose can be represented in three ways:
straight-chain (Fischer form)
cyclic structure (Haworth projection)widely used
(a hemiacetal formed by reaction between the aldehyde group and a hydroxyl
group)
Chair form
17.
Structure of D-GlucoseD -Glucose. (A)
Straight-chain form.
(B) -D -glucose;
Haworth projection.
(C) -D -glucose; chair
form.
18.
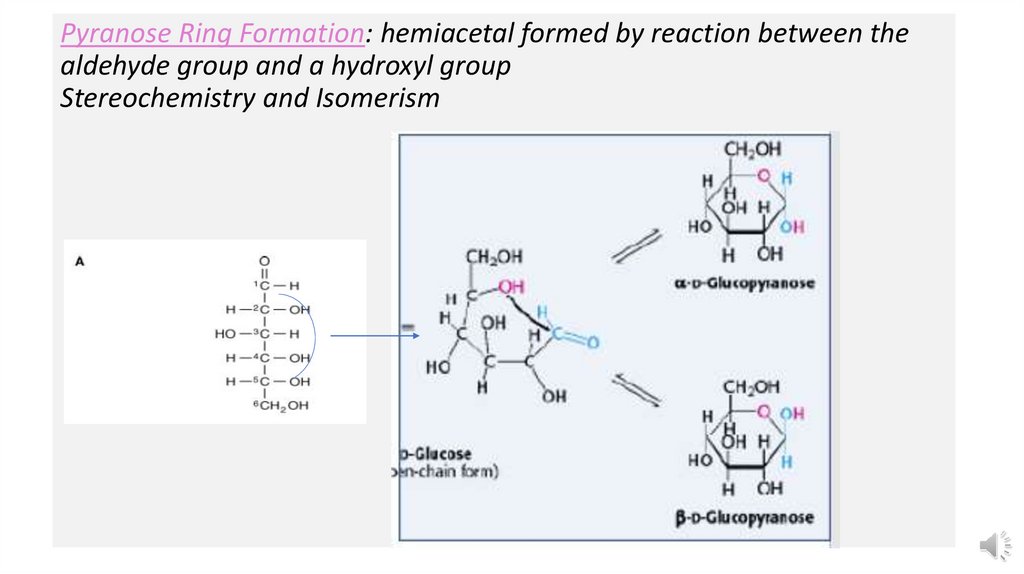
Pyranose Ring Formation: hemiacetal formed by reaction between thealdehyde group and a hydroxyl group
Stereochemistry and Isomerism





 Биология
Биология Химия
Химия








