Похожие презентации:
Quantum Mechanical Model
1.
Denis PribytkinAlexandra Perminova
Ksenia Rokhmistova
Ksenia Vodolazova
1
2.
PART I"CREATION"
2
3.
De Broglie and Bohr:An electron is part of a
complex system, not a
wave, not matter ...
3
4.
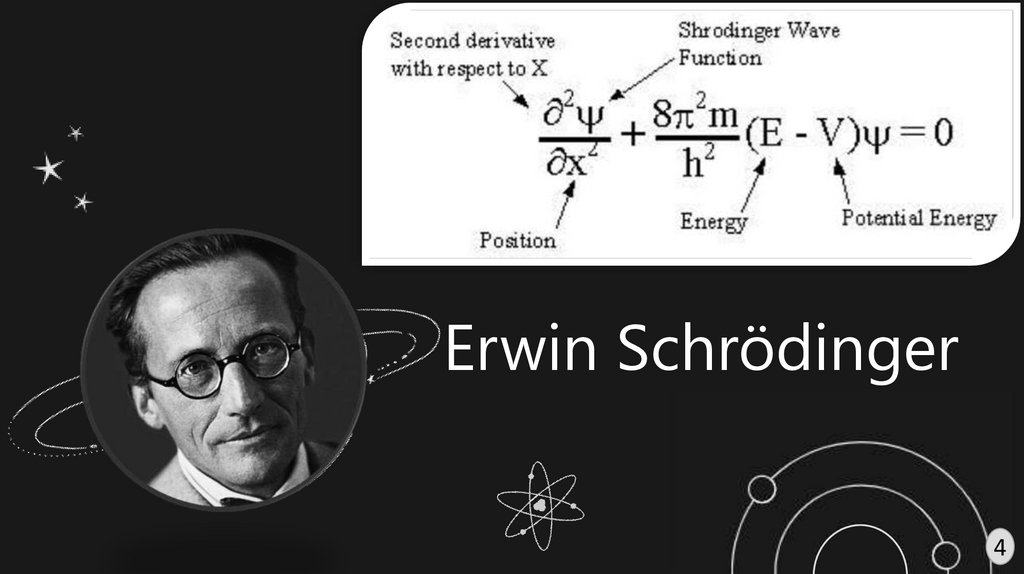
Erwin Schrödinger4
5.
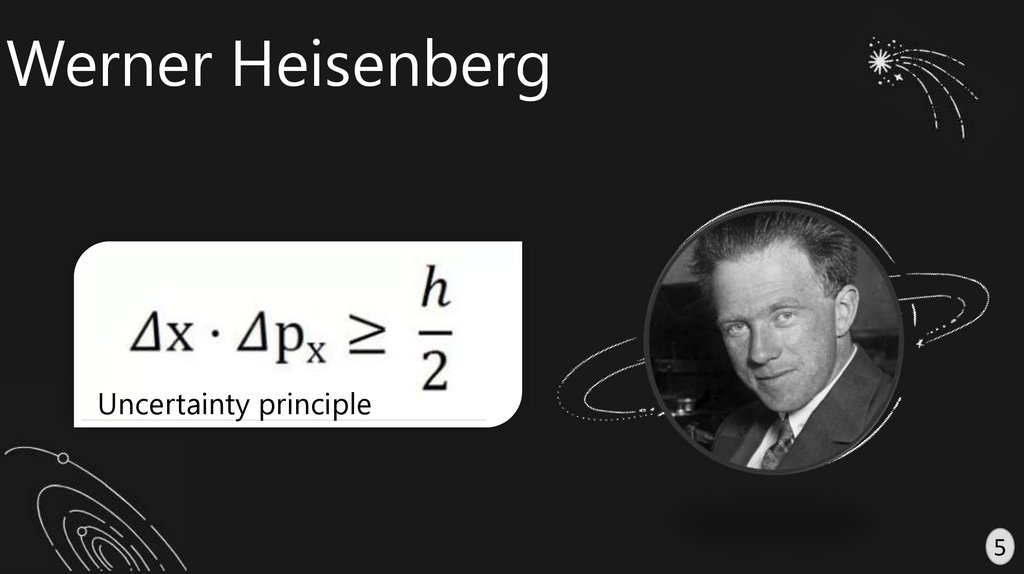
Werner HeisenbergUncertainty principle
5
6.
Planck andEinstein
Matter has rational and irrational
behavior. It is two-dimensional. The
principle of dualism
6
7.
CONCLUSION:Electrons orbit the nucleus in a fixed
circular path termed “orbits” or “shells” or
“energy level.”
The change in energy occurs when the
electrons jump from one energy level
to other an atom, the electrons move from
lower to higher energy level by acquiring
the required energy. However, when an
electron loses energy it moves from higher
to lower energy level.
7
8.
Part IIModel of
God
8
9.
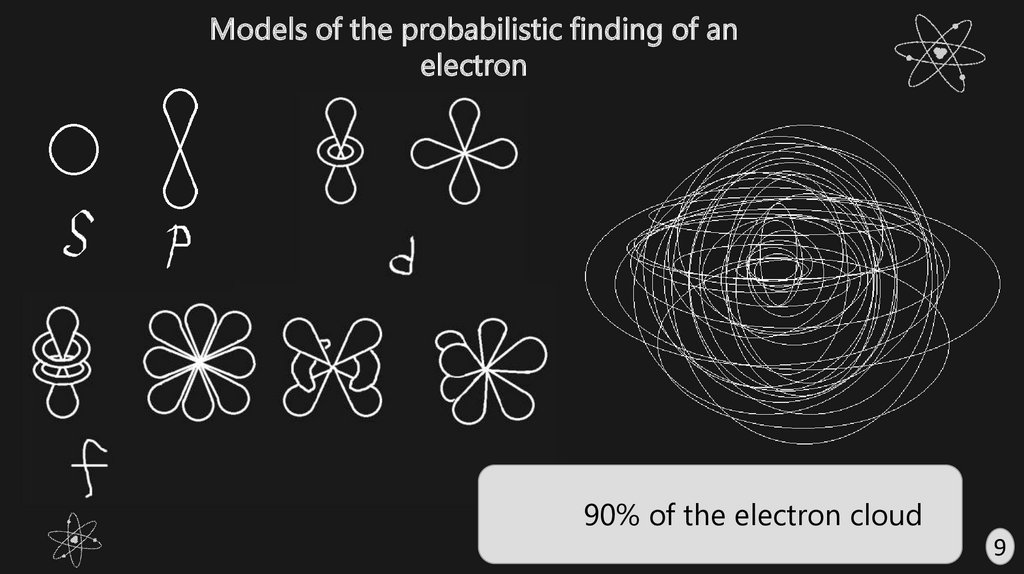
Models of the probabilistic finding of anelectron
90% of the electron cloud
9
10.
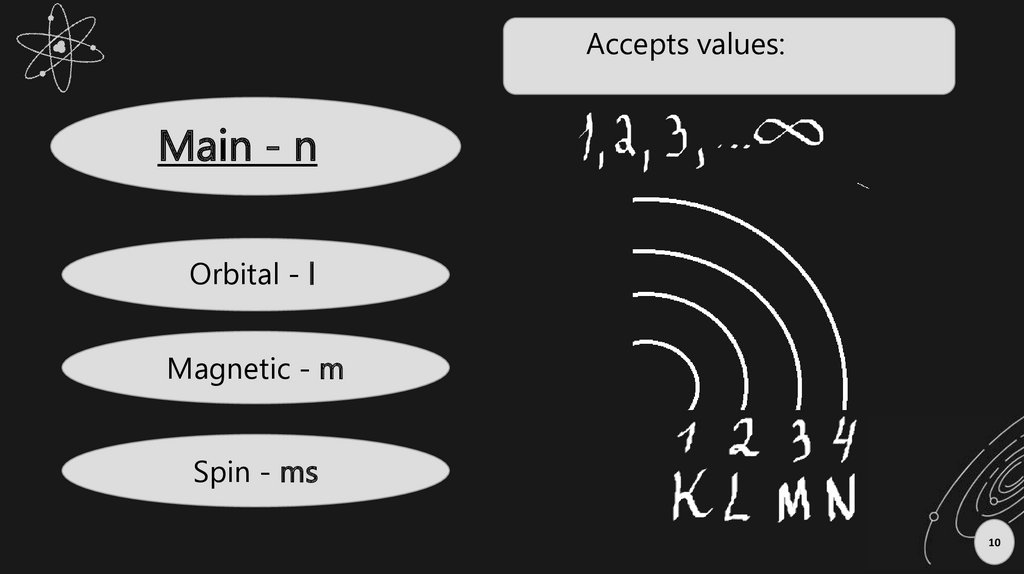
Accepts values:Main - n
Orbital - l
Magnetic - m
Spin - ms
10
11.
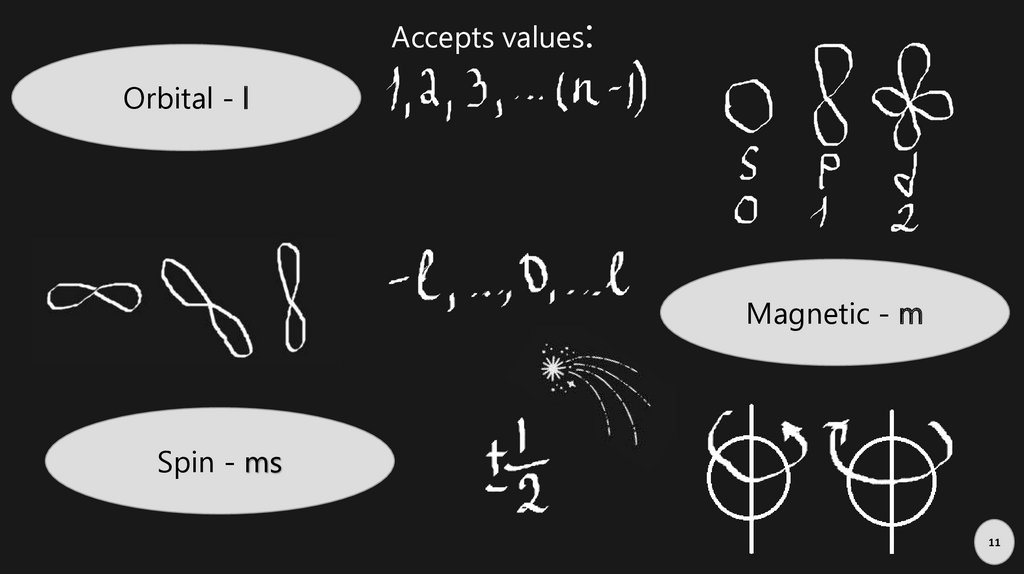
Accepts values:Orbital - l
Magnetic - m
Spin - ms
11
12.
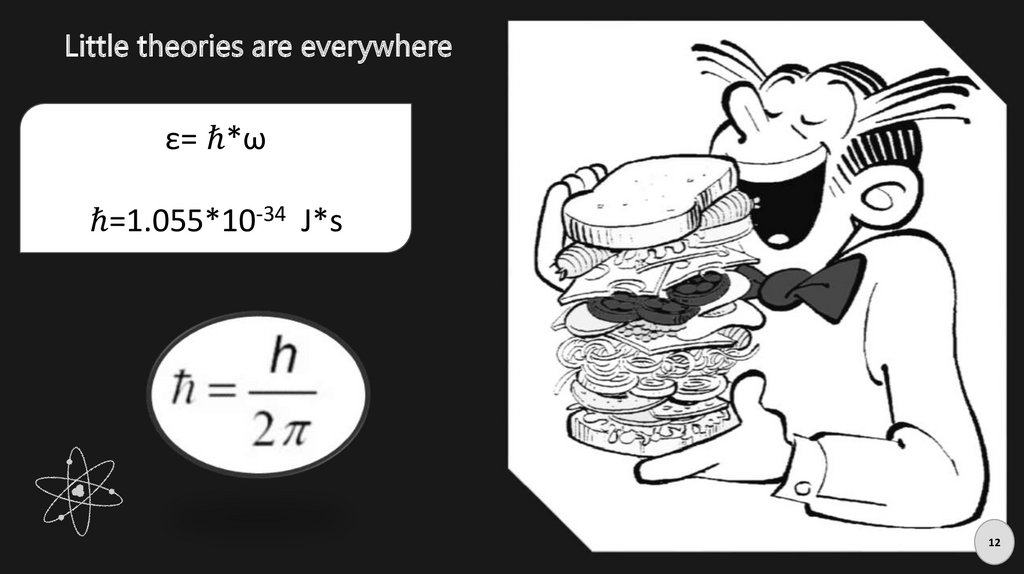
Little theories are everywhereε= ℏ*ω
ℏ=1.055*10-34 J*s
12
13.
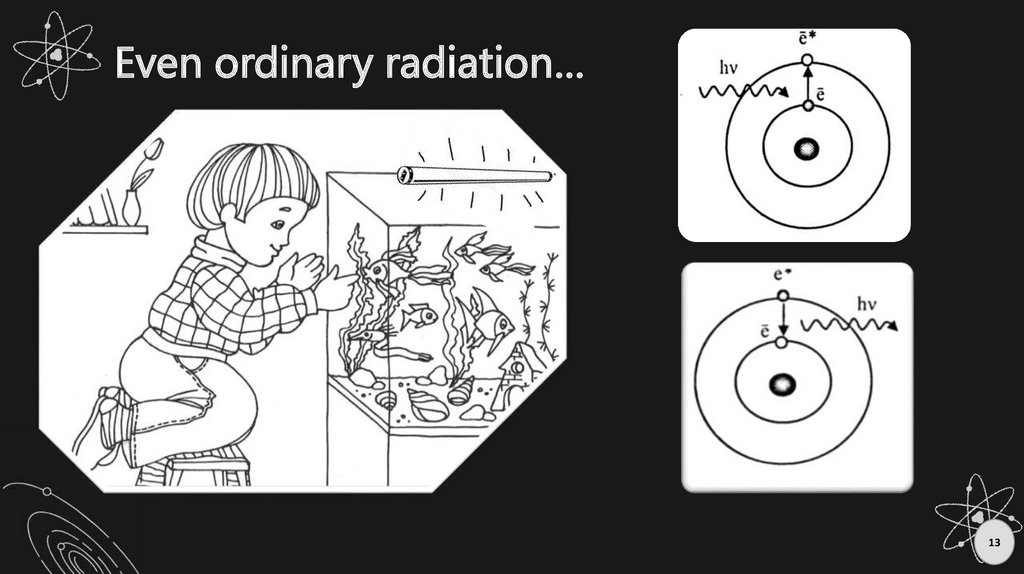
Even ordinary radiation…13
14.
The quantum world everywhere!14
15.
Part IIIThe End of
Eternity
15






 Английский язык
Английский язык








