Похожие презентации:
Atrial fibrillation
1.
ATRIAL FIBRILLATION• Cardiac dysrhythmia (also known as arrhythmia and irregular
heartbeat) is any of a large and heterogeneous group of conditions in
which there is abnormal electrical activity in the heart. The heartbeat
may be too fast or too slow, and may be regular or irregular.
2.
PVC, premature ventricular contractionAPV, atrial premature contraction
PSVT, paroxysmal supraventricular tachycardia
3.
4.
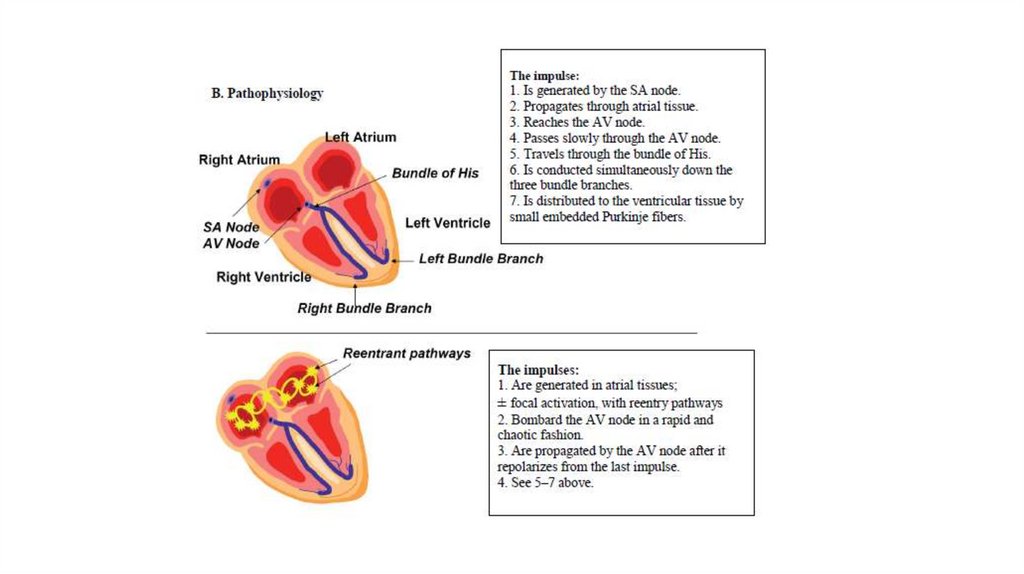
AutomaticityThe sinus node contains pacemaker cells that have spontaneous firing capacity. This
is called normal automaticity.
Abnormal automaticity occurs when other cells start firing spontaneously, resulting
in premature heartbeats.
All cardiac cells have spontaneous firing capacity, but at only a very slow heart rate.
Therefore, during a normal heart rate, they will never have the chance to show off
their firing capacity. However, in pathologic conditions, such as during extreme
bradycardia, other cells can take over and cause for example an AV-nodal heart
rate.
Re-entry
Is the circular propagation of an impulse around 2 interconnected pathways with
different conduction charecterstics and refractory periods
5.
ATRIAL FIBRILLATIONAtrial fibrillation is an irregular and often rapid heart rate that commonly
causes poor blood flow to the body. During atrial fibrillation, the heart's two
upper chambers (the atria) beat chaotically and irregularly — out of
coordination with the two lower chambers (the ventricles) of the heart.
6.
A. Background1 Symptoms
a. Some patients have no symptoms.
b. Most patients have some degree of the following:
i. Palpitations
ii. Chest pain
iii. Dyspnea
iv. Fatigue
v. Light-headedness
7.
2. Classification (more than one of these may exist in a given patient):a. Paroxysmal: Spontaneous self-termination within 7 days of onset
b. Persistent: Lasting more than 7 days (usually require pharmacologic or DC
cardioversion to restore SR)
c. Long-standing persistent: Continuous duration of more than 12 months
d. Permanent: Present all the time, unable to return to normal SR using
pharmacologic or non-pharmacologic options
e. Non-valvular: The absence of rheumatic mitral stenosis, mitral valve repair,
a mechanical or bioprosthetic heart valve
8.
9.
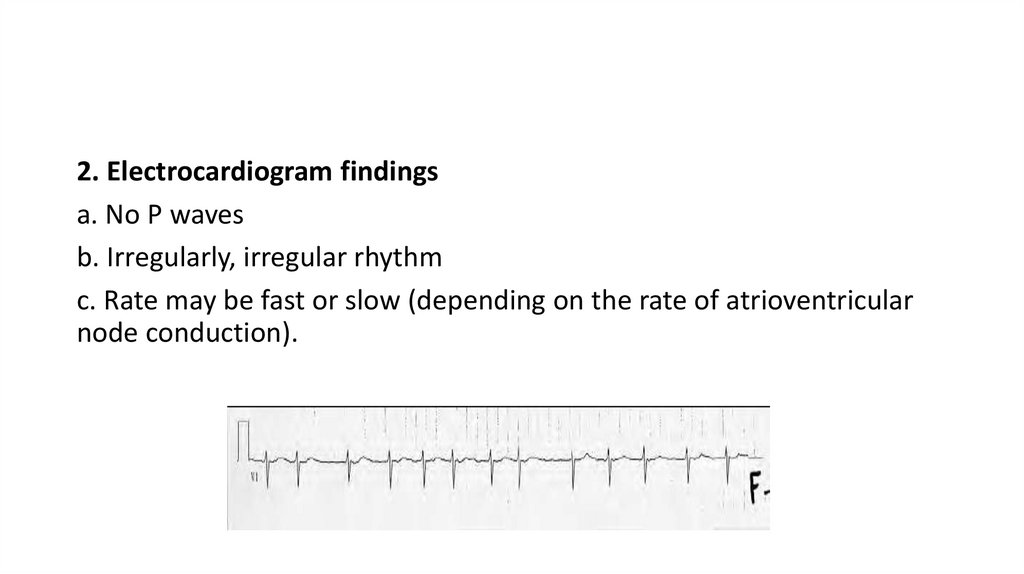
2. Electrocardiogram findingsa. No P waves
b. Irregularly, irregular rhythm
c. Rate may be fast or slow (depending on the rate of atrioventricular
node conduction).
10.
3. Why do these abnormal impulses develop?Potential Causes for Atrial Fibrillation
11.
Atrial DistensionHigh Adrenergic Tone
Chronic hypertension
Alcohol withdrawal
Mitral valve disease
Thyrotoxicosis
Cardiomyopathy
Sepsis
Congenital defects
Binge drinking
Pulmonary hypertension
Cocaine
Acute pulmonary embolus
Amphetamines
Excessive theophylline, caffeine
Surgery
12.
C. Pharmacologic Therapy(a) Rate control (to control high heart rate) plus anticoagulation (according to
CHA2DS2Vasc)
(b) If disabling symptoms, consider adding antiarrhythmic drug therapy or ablation
surgery (important now).
(c) If hemodynamically unstable, synchronized cardioversion recommended
1. Ventricular rate control
a. If patients have a rapid ventricular rate, atrioventricular node blockade is required.
b. Goal HR (resting HR less than 80 beats/minute) is reasonable in symptomatic
patients. A more lenient rate control (resting HR less than 110 beats/minute) may be
reasonable in patients who are asymptomatic and have preserved ejection fraction.
c. These therapies have no effect on the cardioversion of AF:
13.
i. β-Blockers(a) Any agent with β-blockade can be used and dosed to the goal HR.
(b) Labetalol or carvedilol if additional α1 -blockade is desirable (e.g.,
HTN)
(c) Effective for controlling exercise-associated HR increases
(d) β-Blockers may be preferred in patients with a history of MI.
(e) Select carvedilol, metoprolol succinate, or bisoprolol in patients
with HFrEF
(f) Could be used in Wolff-Parkinson-White syndrome but
propafenone and amiodarone are more effective
14.
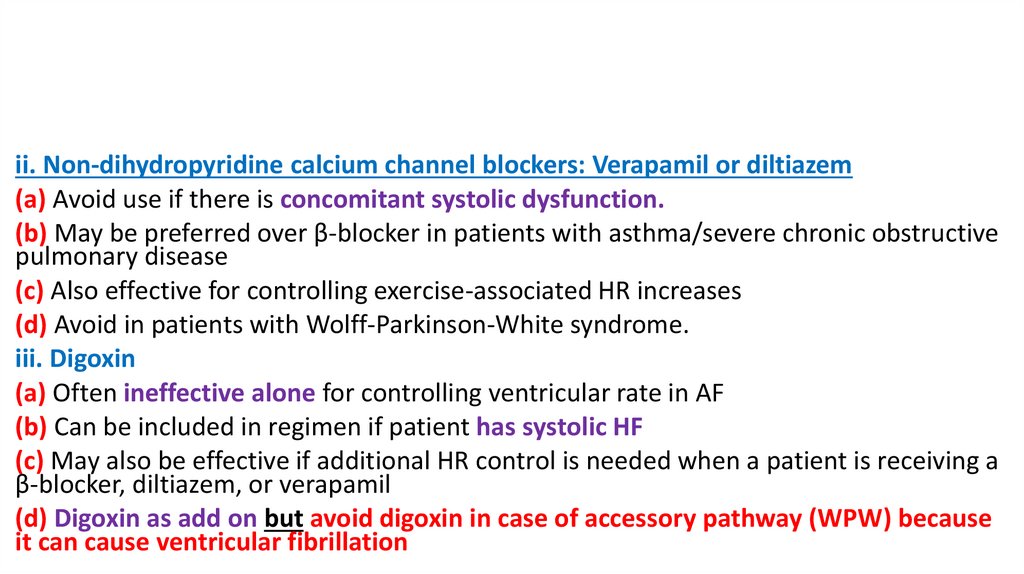
ii. Non-dihydropyridine calcium channel blockers: Verapamil or diltiazem(a) Avoid use if there is concomitant systolic dysfunction.
(b) May be preferred over β-blocker in patients with asthma/severe chronic obstructive
pulmonary disease
(c) Also effective for controlling exercise-associated HR increases
(d) Avoid in patients with Wolff-Parkinson-White syndrome.
iii. Digoxin
(a) Often ineffective alone for controlling ventricular rate in AF
(b) Can be included in regimen if patient has systolic HF
(c) May also be effective if additional HR control is needed when a patient is receiving a
β-blocker, diltiazem, or verapamil
(d) Digoxin as add on but avoid digoxin in case of accessory pathway (WPW) because
it can cause ventricular fibrillation
15.
• iv. Amiodarone (a) May be used for rate control in patients with HFwho do not have an accessory pathway (b) May be used for rate
control in patients who are refractory to other therapies such as βblockers, non-DHP CCBs, and digoxin
16.
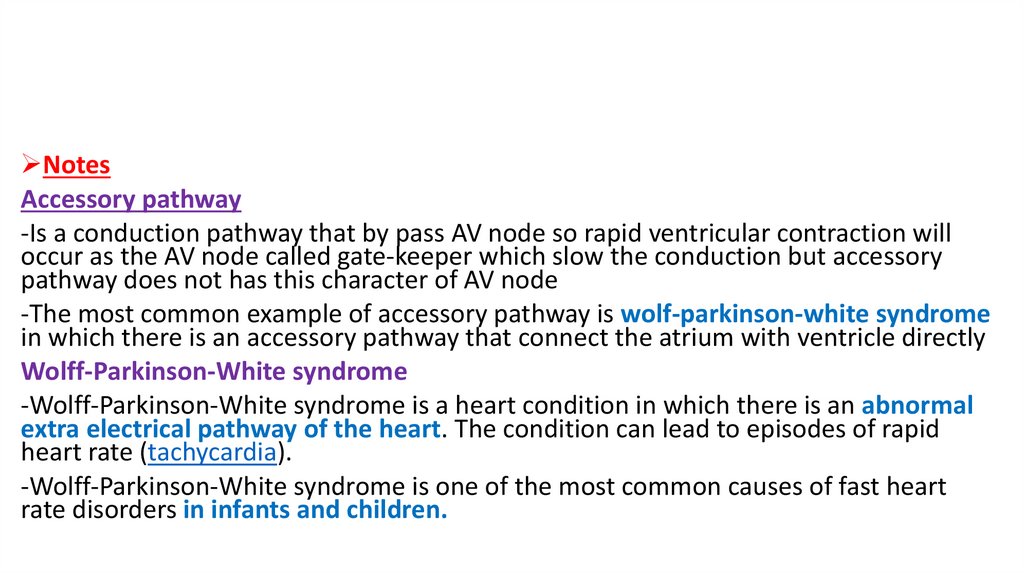
NotesAccessory pathway
-Is a conduction pathway that by pass AV node so rapid ventricular contraction will
occur as the AV node called gate-keeper which slow the conduction but accessory
pathway does not has this character of AV node
-The most common example of accessory pathway is wolf-parkinson-white syndrome
in which there is an accessory pathway that connect the atrium with ventricle directly
Wolff-Parkinson-White syndrome
-Wolff-Parkinson-White syndrome is a heart condition in which there is an abnormal
extra electrical pathway of the heart. The condition can lead to episodes of rapid
heart rate (tachycardia).
-Wolff-Parkinson-White syndrome is one of the most common causes of fast heart
rate disorders in infants and children.
17.
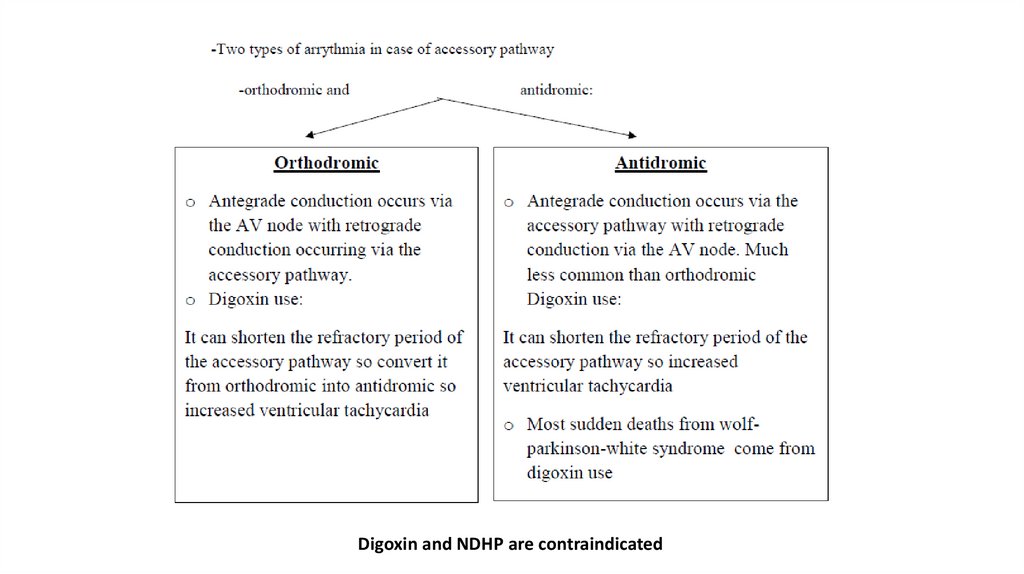
Digoxin and NDHP are contraindicated18.
Orthodromicand antidromic
19.
2. Anticoagulation (Antithrombotic therapy)Risk stratification and treatment determination
Step 1.determine the patient’s risk factors for stroke
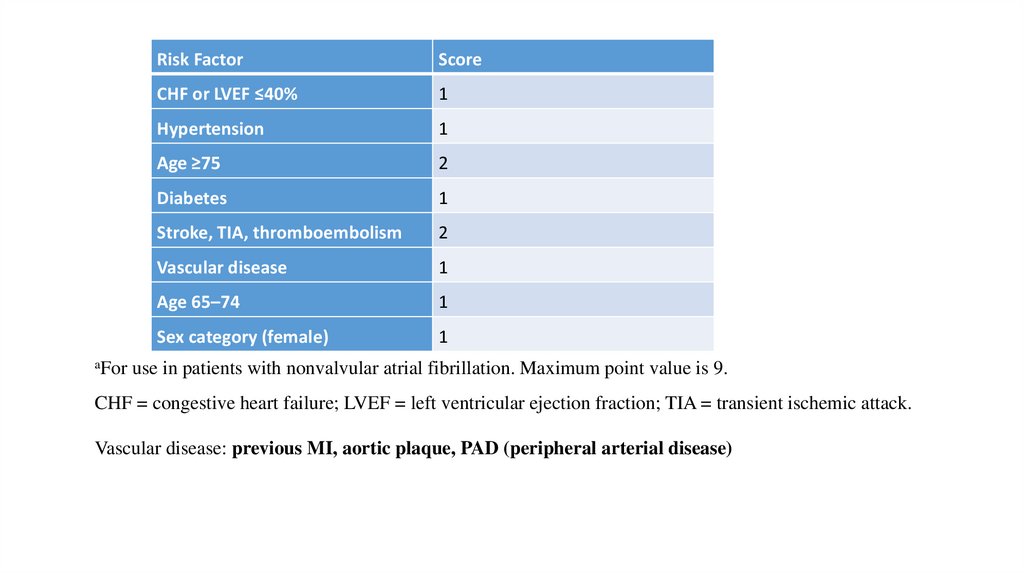
Risk Stratification for Antithrombotic Therapy Using the CHA2DS2VASc
Score
20.
Risk FactorScore
CHF or LVEF ≤40%
1
Hypertension
1
Age ≥75
2
Diabetes
1
Stroke, TIA, thromboembolism
2
Vascular disease
1
Age 65–74
1
Sex category (female)
1
aFor use in patients with nonvalvular atrial fibrillation. Maximum point value is 9.
CHF = congestive heart failure; LVEF = left ventricular ejection fraction; TIA = transient ischemic attack.
Vascular disease: previous MI, aortic plaque, PAD (peripheral arterial disease)
21.
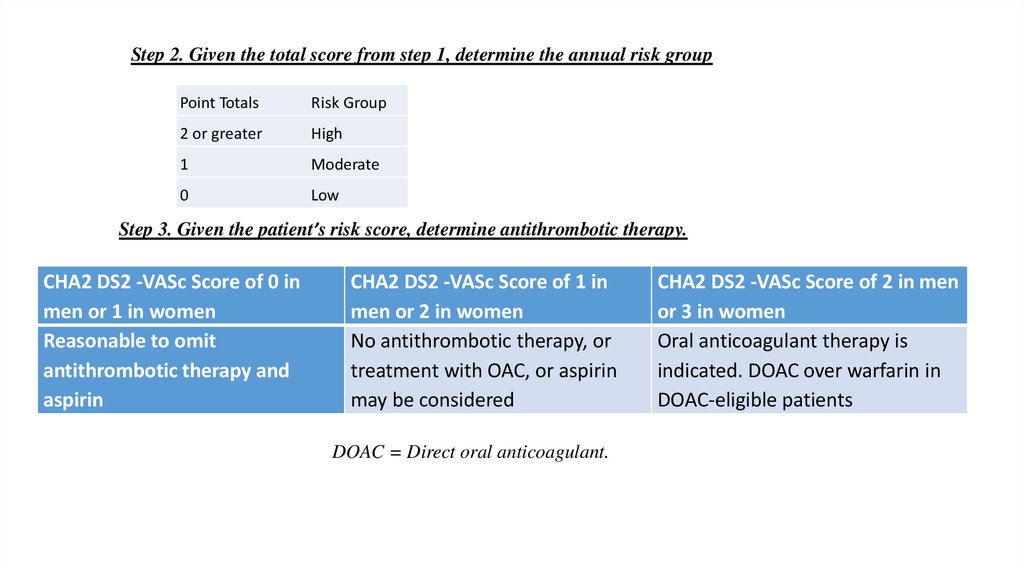
Step 2. Given the total score from step 1, determine the annual risk groupPoint Totals
Risk Group
2 or greater
High
1
Moderate
0
Low
Step 3. Given the patient’s risk score, determine antithrombotic therapy.
CHA2 DS2 -VASc Score of 0 in
men or 1 in women
Reasonable to omit
antithrombotic therapy and
aspirin
CHA2 DS2 -VASc Score of 1 in
men or 2 in women
No antithrombotic therapy, or
treatment with OAC, or aspirin
may be considered
DOAC = Direct oral anticoagulant.
CHA2 DS2 -VASc Score of 2 in men
or 3 in women
Oral anticoagulant therapy is
indicated. DOAC over warfarin in
DOAC-eligible patients
22.
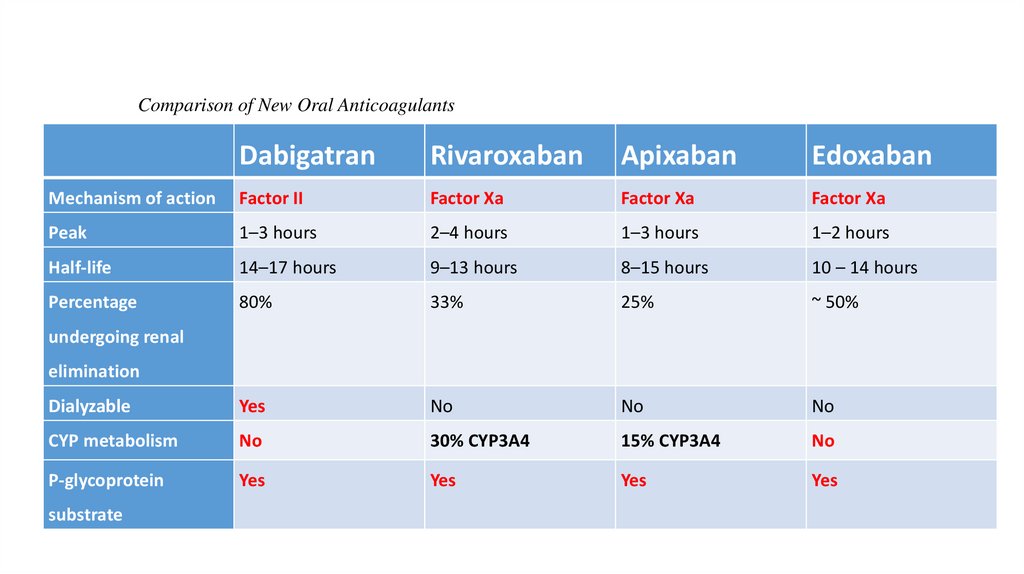
Comparison of New Oral AnticoagulantsDabigatran
Rivaroxaban
Apixaban
Edoxaban
Mechanism of action
Factor II
Factor Xa
Factor Xa
Factor Xa
Peak
1–3 hours
2–4 hours
1–3 hours
1–2 hours
Half-life
14–17 hours
9–13 hours
8–15 hours
10 – 14 hours
Percentage
80%
33%
25%
~ 50%
Dialyzable
Yes
No
No
No
CYP metabolism
No
30% CYP3A4
15% CYP3A4
No
P-glycoprotein
Yes
Yes
Yes
Yes
undergoing renal
elimination
substrate
23.
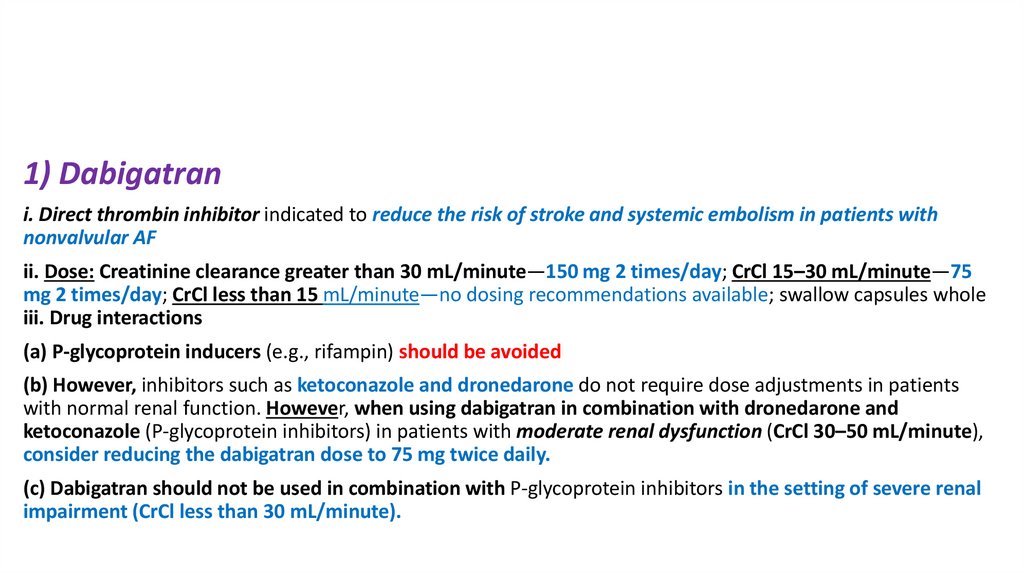
1) Dabigatrani. Direct thrombin inhibitor indicated to reduce the risk of stroke and systemic embolism in patients with
nonvalvular AF
ii. Dose: Creatinine clearance greater than 30 mL/minute—150 mg 2 times/day; CrCl 15–30 mL/minute—75
mg 2 times/day; CrCl less than 15 mL/minute—no dosing recommendations available; swallow capsules whole
iii. Drug interactions
(a) P-glycoprotein inducers (e.g., rifampin) should be avoided
(b) However, inhibitors such as ketoconazole and dronedarone do not require dose adjustments in patients
with normal renal function. However, when using dabigatran in combination with dronedarone and
ketoconazole (P-glycoprotein inhibitors) in patients with moderate renal dysfunction (CrCl 30–50 mL/minute),
consider reducing the dabigatran dose to 75 mg twice daily.
(c) Dabigatran should not be used in combination with P-glycoprotein inhibitors in the setting of severe renal
impairment (CrCl less than 30 mL/minute).
24.
Idarucizumab is the antidote of dabigatranoIdarucizumab is monoclonal antibody that bind to
dabigatran 350 times higher affinity than thrombin
Andexanet alph is the antidote for rivaroxaban, edoxaban
and apixaban
oAndexanet alph is a modified recombinant factor Xa that
strongly binds to rivaroxaban, edoxaban or apixaban
25.
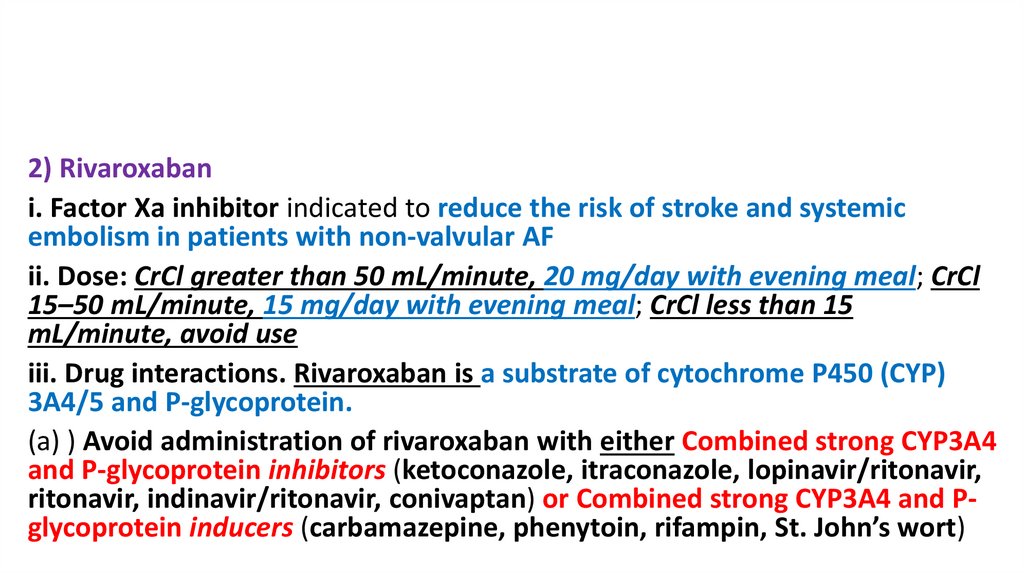
2) Rivaroxabani. Factor Xa inhibitor indicated to reduce the risk of stroke and systemic
embolism in patients with non-valvular AF
ii. Dose: CrCl greater than 50 mL/minute, 20 mg/day with evening meal; CrCl
15–50 mL/minute, 15 mg/day with evening meal; CrCl less than 15
mL/minute, avoid use
iii. Drug interactions. Rivaroxaban is a substrate of cytochrome P450 (CYP)
3A4/5 and P-glycoprotein.
(a) ) Avoid administration of rivaroxaban with either Combined strong CYP3A4
and P-glycoprotein inhibitors (ketoconazole, itraconazole, lopinavir/ritonavir,
ritonavir, indinavir/ritonavir, conivaptan) or Combined strong CYP3A4 and Pglycoprotein inducers (carbamazepine, phenytoin, rifampin, St. John’s wort)
26.
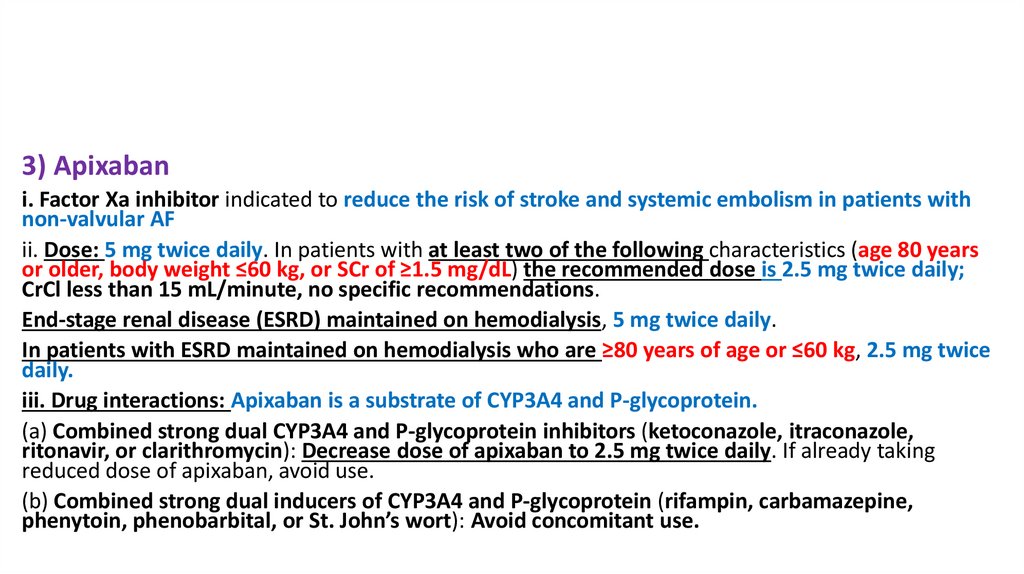
3) Apixabani. Factor Xa inhibitor indicated to reduce the risk of stroke and systemic embolism in patients with
non-valvular AF
ii. Dose: 5 mg twice daily. In patients with at least two of the following characteristics (age 80 years
or older, body weight ≤60 kg, or SCr of ≥1.5 mg/dL) the recommended dose is 2.5 mg twice daily;
CrCl less than 15 mL/minute, no specific recommendations.
End-stage renal disease (ESRD) maintained on hemodialysis, 5 mg twice daily.
In patients with ESRD maintained on hemodialysis who are ≥80 years of age or ≤60 kg, 2.5 mg twice
daily.
iii. Drug interactions: Apixaban is a substrate of CYP3A4 and P-glycoprotein.
(a) Combined strong dual CYP3A4 and P-glycoprotein inhibitors (ketoconazole, itraconazole,
ritonavir, or clarithromycin): Decrease dose of apixaban to 2.5 mg twice daily. If already taking
reduced dose of apixaban, avoid use.
(b) Combined strong dual inducers of CYP3A4 and P-glycoprotein (rifampin, carbamazepine,
phenytoin, phenobarbital, or St. John’s wort): Avoid concomitant use.
27.
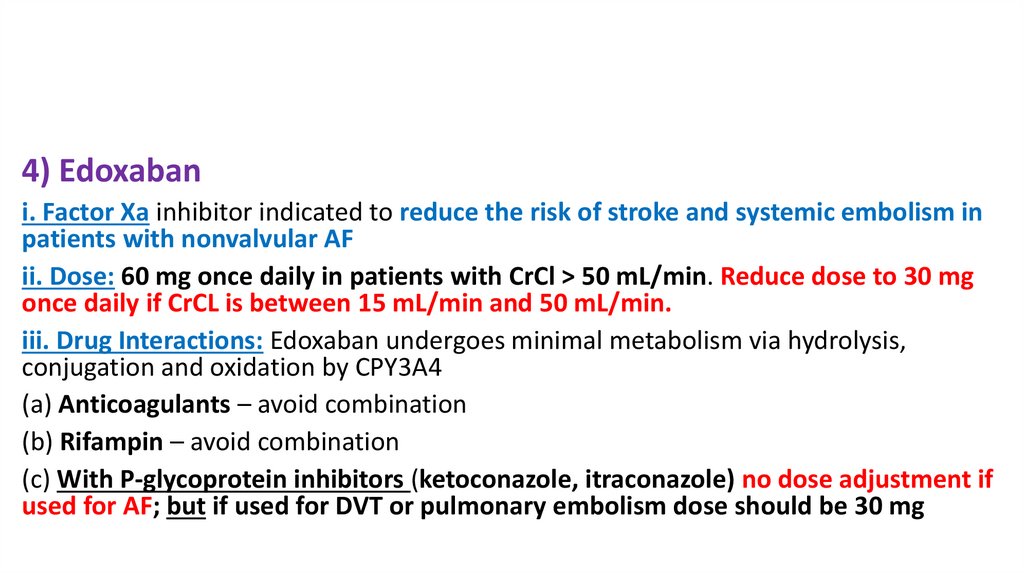
4) Edoxabani. Factor Xa inhibitor indicated to reduce the risk of stroke and systemic embolism in
patients with nonvalvular AF
ii. Dose: 60 mg once daily in patients with CrCl > 50 mL/min. Reduce dose to 30 mg
once daily if CrCL is between 15 mL/min and 50 mL/min.
iii. Drug Interactions: Edoxaban undergoes minimal metabolism via hydrolysis,
conjugation and oxidation by CPY3A4
(a) Anticoagulants – avoid combination
(b) Rifampin – avoid combination
(c) With P-glycoprotein inhibitors (ketoconazole, itraconazole) no dose adjustment if
used for AF; but if used for DVT or pulmonary embolism dose should be 30 mg
28.
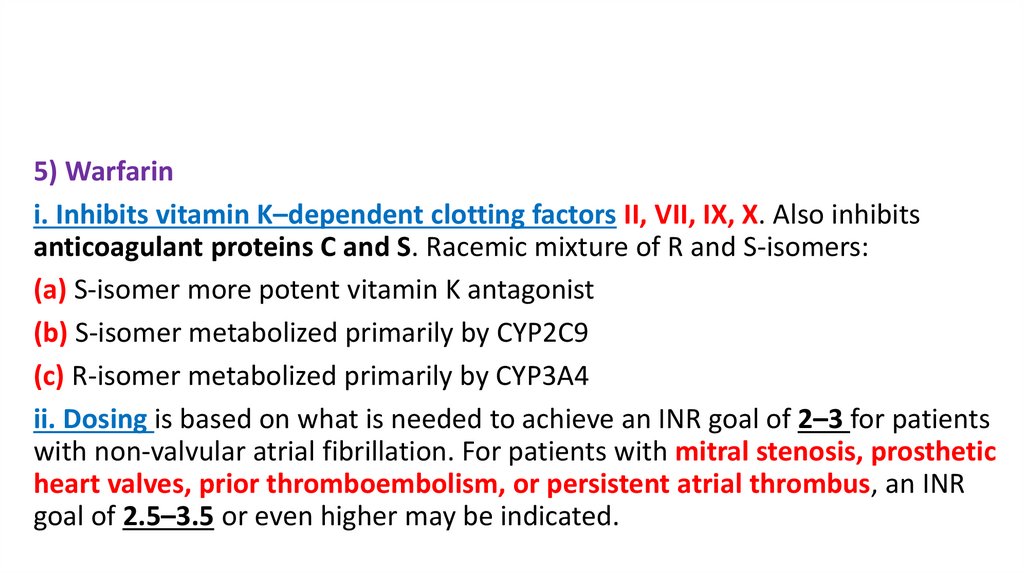
5) Warfarini. Inhibits vitamin K–dependent clotting factors II, VII, IX, X. Also inhibits
anticoagulant proteins C and S. Racemic mixture of R and S-isomers:
(a) S-isomer more potent vitamin K antagonist
(b) S-isomer metabolized primarily by CYP2C9
(c) R-isomer metabolized primarily by CYP3A4
ii. Dosing is based on what is needed to achieve an INR goal of 2–3 for patients
with non-valvular atrial fibrillation. For patients with mitral stenosis, prosthetic
heart valves, prior thromboembolism, or persistent atrial thrombus, an INR
goal of 2.5–3.5 or even higher may be indicated.
29.
iii. Initial starting dose is usually 5 mg/day.(a) Half-lives of vitamin K–dependent clotting factor VII, 6 hours; factor IX, 24
hours; factor X, 36 hours; factor II, 72 hours
(b) Adjusting dose: Watch for trends; remember that the INR seen today is the
result of the doses given in the past 4–5 days. Takes 5–7 days to reach full
effect, given the half-life of factor II
(c) In severe case overlap with heparin (LMWH or UH) until INR reach 2-3 (5-7
days) to prevent initial thrombosis that may occur due to inhibition of S and C
of warfarin and also until the full effect of warfarin reached.
(d) If INR is out of therapeutic range, increase or decrease cumulative weekly
warfarin dose by 5%–20% depending on INR; if INR is high (generally above
4.5), may hold one or two doses and resume at a lower dose
30.
iv. Drug interactions(a) Reduced warfarin absorption (e.g., cholestyramine, sucralfate)
(b) Enzyme induction (decreases INR and warfarin effects)
(1) CYP3A4 inducers (e.g., rifampin, carbamazepine, phenobarbital, St. John’s
wort)
31.
(c) Enzyme inhibition (increases INR and warfarin effects)(1) S-warfarin (CYP2C9 inhibitors) (e.g., metronidazole, trimethoprim/sulfamethoxazole,
fluconazole, isoniazid, fluoxetine, sertraline, amiodarone, clopidogrel, lovastatin)
(2) R-warfarin (CYP3A3/4/5 inhibitors) (e.g., omeprazole, metronidazole, “azole”
antifungals, isoniazid, fluoxetine, sertraline, amiodarone, lovastatin clarithromycin,
erythromycin, nefazodone, cyclosporine, grapefruit juice, ciprofloxacin, norfloxacin,
protease inhibitors, diltiazem, verapamil, ,)
(d) Drugs with antiplatelet effects (e.g., gingko, garlic, aspirin, NSAIDs, selective
serotonin reuptake inhibitors), increase its effect.
(e) Drugs that reduce warfarin clearance (e.g., propafenone); increase INR
(f) Drugs that increase the degradation of clotting factors (e.g., levothyroxine);
increase INR
32.
v. Bleeding: Epistaxis, hematuria, GI hemorrhage, bleeding gums, easy bruisingoften occurs with therapeutic INR.
(a) Minor hemorrhage increased with therapeutic warfarin therapy
(b) Major hemorrhage not increased with warfarin therapy at INR 2–3
(c) Risk of intracranial hemorrhage increased with INR greater than 4
3. Rhythm control:
Maintaining SR offers no advantage over controlling the ventricular rate. However,
in specific patients with intractable and intolerable symptoms (dyspnea,
palpitations, and exercise intolerance) despite adequate rate control or in patients
for whom adequate ventricular rate control cannot be achieved, restoration and
maintenance of SR may be desirable
Options for rhythm control in patients with paroxysmal and persistent atrial fibrillation
33.
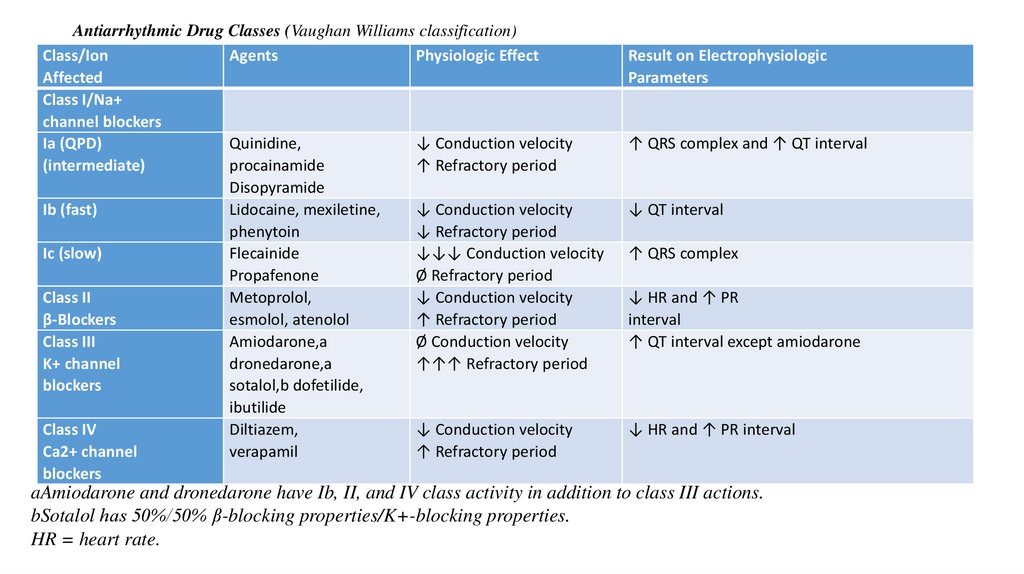
Antiarrhythmic Drug Classes (Vaughan Williams classification)Class/Ion
Agents
Physiologic Effect
Affected
Class I/Na+
channel blockers
Ia (QPD)
Quinidine,
↓ Conduction velocity
(intermediate)
procainamide
↑ Refractory period
Disopyramide
Ib (fast)
Lidocaine, mexiletine,
↓ Conduction velocity
phenytoin
↓ Refractory period
Ic (slow)
Flecainide
↓↓↓ Conduction velocity
Propafenone
Ø Refractory period
Class II
Metoprolol,
↓ Conduction velocity
β-Blockers
esmolol, atenolol
↑ Refractory period
Class III
Amiodarone,a
Ø Conduction velocity
K+ channel
dronedarone,a
↑↑↑ Refractory period
blockers
sotalol,b dofetilide,
ibutilide
Class IV
Diltiazem,
↓ Conduction velocity
Ca2+ channel
verapamil
↑ Refractory period
blockers
Result on Electrophysiologic
Parameters
↑ QRS complex and ↑ QT interval
↓ QT interval
↑ QRS complex
↓ HR and ↑ PR
interval
↑ QT interval except amiodarone
↓ HR and ↑ PR interval
aAmiodarone and dronedarone have Ib, II, and IV class activity in addition to class III actions.
bSotalol has 50%/50% β-blocking properties/K+-blocking properties.
HR = heart rate.
34.
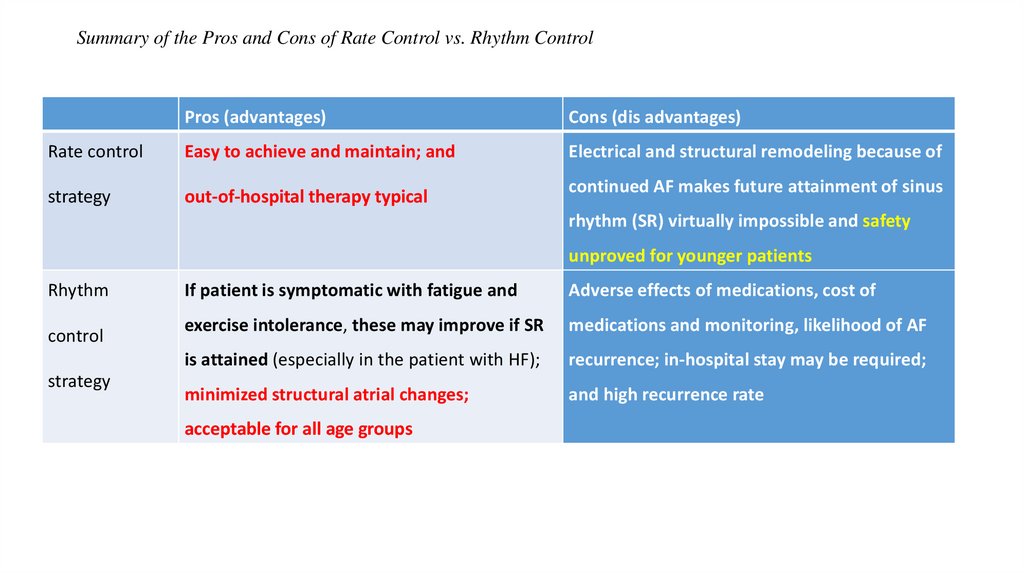
35.
Summary of the Pros and Cons of Rate Control vs. Rhythm ControlPros (advantages)
Cons (dis advantages)
Rate control
Easy to achieve and maintain; and
Electrical and structural remodeling because of
strategy
out-of-hospital therapy typical
continued AF makes future attainment of sinus
rhythm (SR) virtually impossible and safety
unproved for younger patients
Rhythm
control
strategy
If patient is symptomatic with fatigue and
Adverse effects of medications, cost of
exercise intolerance, these may improve if SR
medications and monitoring, likelihood of AF
is attained (especially in the patient with HF);
recurrence; in-hospital stay may be required;
minimized structural atrial changes;
and high recurrence rate
acceptable for all age groups
36.
Oral antiarrhythmics to induce/maintain sinus rhythmi. Class III antiarrhythmics
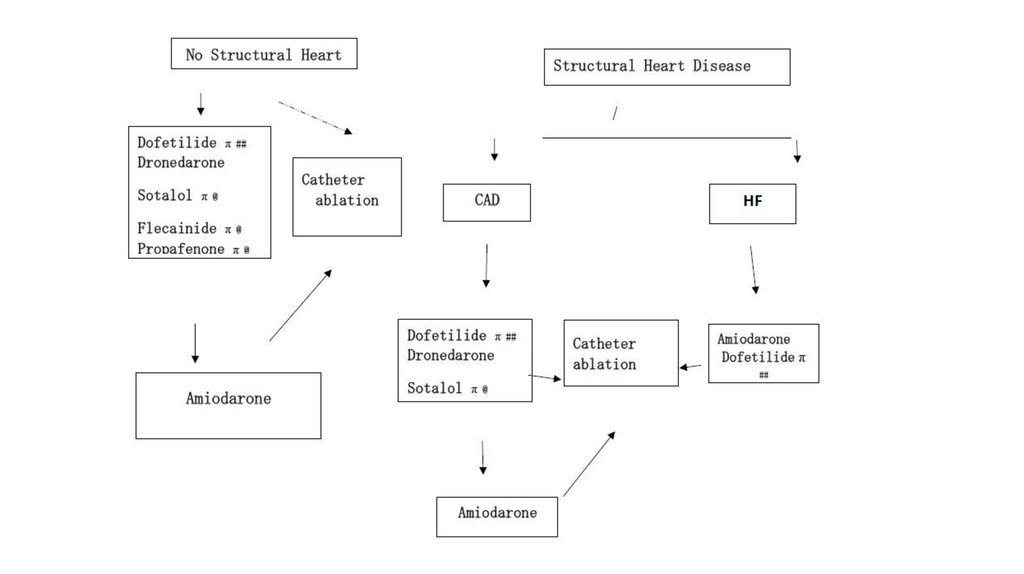
(a) Amiodarone: 85%–95% efficacy
(1) Oral loading dose required (e.g., 400 mg 2 or 3 times per day for 2 weeks and then 400 mg/day for 4 weeks,
followed by a 200-mg/day maintenance dose). Achieving a loading dose of 10 g is desirable. Many different
regimens exist.
(2) Long half-life of about 60 days
(3) In addition, has atrioventricular nodal blocking properties; may help control HR if AF recurs
(4) Hepatically metabolized; inhibitor of cytochrome P450 (CYP) enzymes and P-glycoprotein
(5) Drug interactions (many)
(A) Digoxin—Increased digoxin exposure. Lower initial digoxin dose by 50%
(B) Warfarin—Increased warfarin exposure. Lower warfarin dose by 25%– 30%
(C) Simvastatin—Increased simvastatin exposure. Do not exceed dose of 20 mg/day.
(D) Lovastatin: Increased lovastatin exposure. Do not exceed dose of 40 mg/day.
(E) β-Blockers, non-DHP CCBs, clonidine, ivabradine: Additive bradycardia
37.
(6) Extensive monitoring for non-cardiac adverse effects(A) Liver function tests: Baseline and every 6 months
(B) Thyroid function tests: Baseline and every 6 months
(C) Chest radiography: Baseline and annually
(D) Pulmonary function tests. Baseline and for unexplained cough/dyspnea, chest radiographic abnormalities
or clinical suspicion. Discontinue if pulmonary fibrosis occurs.
(E) ECG: Periodically
(F) Ophthalmologic examination: For symptoms of visual impairment. Discontinue if optic neuritis occurs.
(G) Skin toxicities (necrosis): “Blue skin” syndrome and sunburn (rare skin necrosis in which there is a
paradoxical blood clotting. If so; stop warfarin and initiate heparin or new oral anticoagulant.
(H) Neurologic toxicity: Tremor, neuropathy
(I) Nausea, vomiting
(J) Adverse effects may require increased monitoring, dose reduction, or drug discontinuation
38.
(b) Class Ic antiarrhythmics: 80% - 90% efficacy(i) Flecainide and propafenone can be considered first-line therapies
for patients without structural heart disease.
Propafenone also displays some nonselective β-blocking properties.
(ii) Concomitant AV nodal blocking agent (e.g., β-blocker or non-DHP
CCB) typically required
(iii) Contraindicated in patients with structural heart disease
(including CHD, HF, left ventricular hypertrophy, and valvular heart
disease)
39.
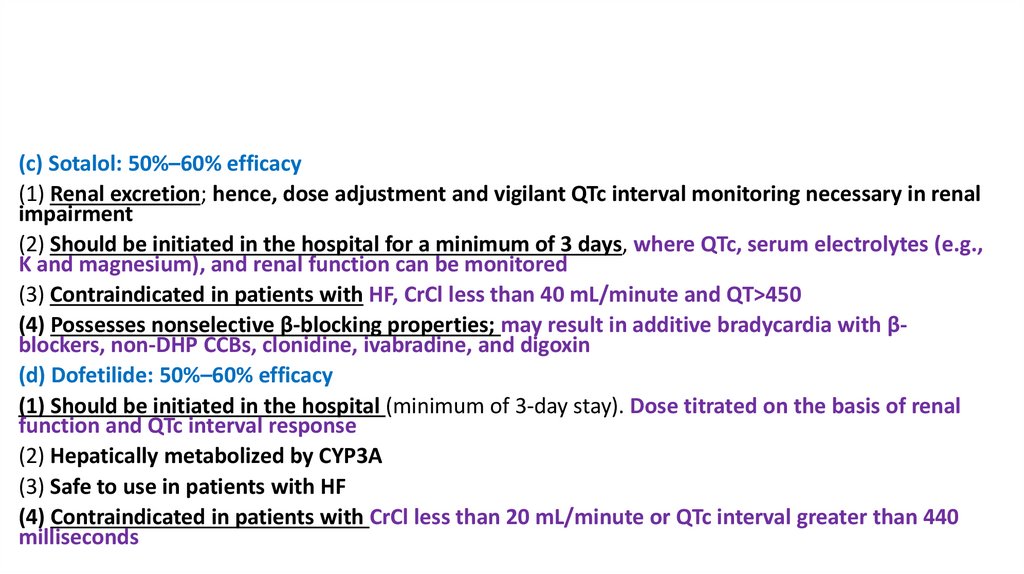
(c) Sotalol: 50%–60% efficacy(1) Renal excretion; hence, dose adjustment and vigilant QTc interval monitoring necessary in renal
impairment
(2) Should be initiated in the hospital for a minimum of 3 days, where QTc, serum electrolytes (e.g.,
K and magnesium), and renal function can be monitored
(3) Contraindicated in patients with HF, CrCl less than 40 mL/minute and QT>450
(4) Possesses nonselective β-blocking properties; may result in additive bradycardia with βblockers, non-DHP CCBs, clonidine, ivabradine, and digoxin
(d) Dofetilide: 50%–60% efficacy
(1) Should be initiated in the hospital (minimum of 3-day stay). Dose titrated on the basis of renal
function and QTc interval response
(2) Hepatically metabolized by CYP3A
(3) Safe to use in patients with HF
(4) Contraindicated in patients with CrCl less than 20 mL/minute or QTc interval greater than 440
milliseconds
40.
• QT is the time from the start of the Q wave to the end of Twave (time from cardiac ventricles start to contract to thetime when they finish relaxation)
• QTc is correction for heart rate
• Normal range for adult men 350-450 ms
• Normal range for adult women 360-460 ms
• Abnormal QTc in male >450; in female > 470ms
• QT prolongation leads to torsades de pointes arrhythmia
41.
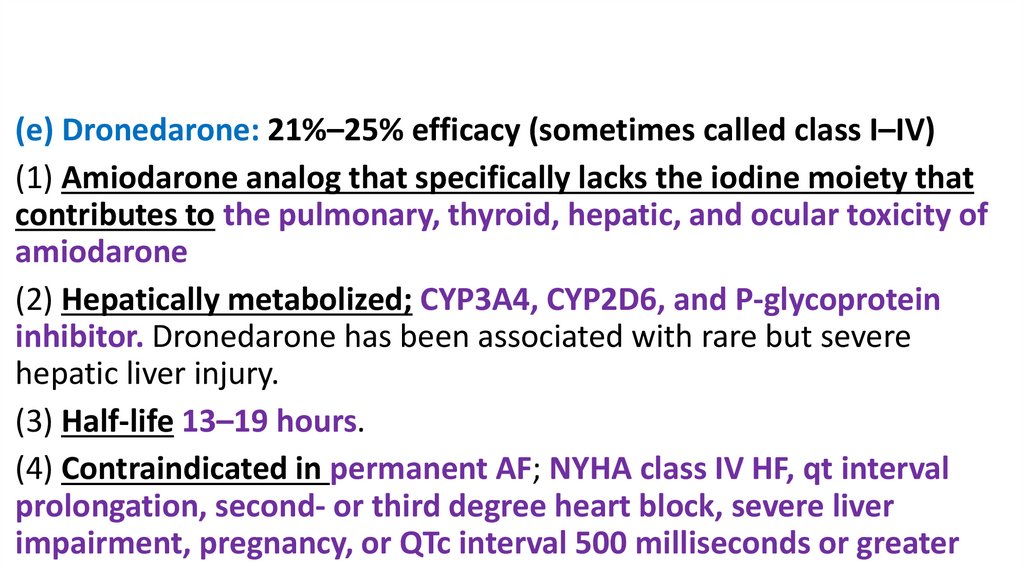
(e) Dronedarone: 21%–25% efficacy (sometimes called class I–IV)(1) Amiodarone analog that specifically lacks the iodine moiety that
contributes to the pulmonary, thyroid, hepatic, and ocular toxicity of
amiodarone
(2) Hepatically metabolized; CYP3A4, CYP2D6, and P-glycoprotein
inhibitor. Dronedarone has been associated with rare but severe
hepatic liver injury.
(3) Half-life 13–19 hours.
(4) Contraindicated in permanent AF; NYHA class IV HF, qt interval
prolongation, second- or third degree heart block, severe liver
impairment, pregnancy, or QTc interval 500 milliseconds or greater
42.
(5) Drug interactions(A) Digoxin: Increased digoxin exposure; lower dose of digoxin by 50%
(B) β-Blockers, non-DHP CCBs, and clonidine: Excessive bradycardia; initiate
these drugs at lowest dose. Diltiazem and verapamil can increase dronedarone
exposure; therefore, monitor ECG.
(C) Dabigatran: In patients with moderate renal impairment (CrCl 30–50 mL/minute/1.73 m2), dronedarone increases dabigatran exposure, dabigatran dose
reduction to 75 mg twice daily is recommended.
(D) Statins: Increased statin exposure. Limit dose of simvastatin to 10 mg/day
and lovastatin to 20 mg/day.
(E) Cyclosporine, tacrolimus, sirolimus: Increased exposure of these agents
(F) CYP3A4 inhibitors: AVOID.
43.
4. Cardioversiona
Electrical cardioversion (low-energy cardioversion, sedation highly desirable
a. If cardioversion is attempted (electric or pharmacologic), the absence of atrial
thrombi must be ensured:
i. Thrombi present plus cardioversion = 91% stroke rate
ii. Without anticoagulation (caused by decreased or stagnant blood flow in the
atria) we can found that:
(a) Atrial fibrillation for greater than 48 hours = 15% rate of atrial thrombus
(b) Atrial fibrillation for greater than 72 hours = 30% rate of atrial thrombus
44.
b. Ensure safe cardioversion by either:(a) Transesophageal echocardiogram (TEE) to visualize the atria, or
(b) Three or more weeks of therapeutic anticoagulation
(1) INR 2.0-3.0 if warfarin is selected
(2) DOACs may also be used
(3) Continue anticoagulation for at least four weeks after cardioversion with either:
(a) Warfarin or
(b) A DOAC
pharmacologic cardioversion:
Flecainide, propafenone, dofetilide, ibutilide, or amiodarone could be used
45.
4. Cardioversiona. electrical cardioversion
Anticoagulation Strategies Surrounding Cardioversion of AFa
Unstable
Synchronized Cardioversion, Anticoagulation Immediately Beforehand
Anticoagulate for ≥4 wk after cardioversion with warfarin or a DOAC if AF ≥ 48 hr or if
duration is unknown
Stable, duration < 48 hr
b
• Anticoagulate at presentation and continue through cardioversion
– LMWH or UFH at full treatment doses
• Anticoagulate for at least 4 wk afterward, regardless of baseline risk of stroke
Stable, duration unknown or > 48 hr
• Anticoagulate for 3 wk before cardioversion
– VKA with INR 2.0–3.0, LMWH at full treatment dose, or dabigatran
Anticoagulate for 4 wk after cardioversion, regardless of CHA2DS2-VASc score
TEE-guided cardioversion for stable,
• TEE-guided therapy with abbreviated anticoagulation before cardioversion
duration unknown or > 48 hr
– LMWH or UFH at full treatment doses should be initiated at the time of TEE, and
cardioversion should be performed within 24 hr of TEE if no thrombus is seen
• Anti-coagulate for 4 wk after cardioversion regardless of baseline risk of stroke
46.
5. Surgical proceduresa. Atrioventricular nodal ablation: Ablate
atrioventricular node and chronically pace the
ventricles.
b. Pulmonary vein ablation: Ablates the origin of the
abnormal atrial foci, which is often near the
pulmonary vein–atrial tissue intersection.



























 Медицина
Медицина