Похожие презентации:
Heart failure
1.
HEART FAILURE2.
3.
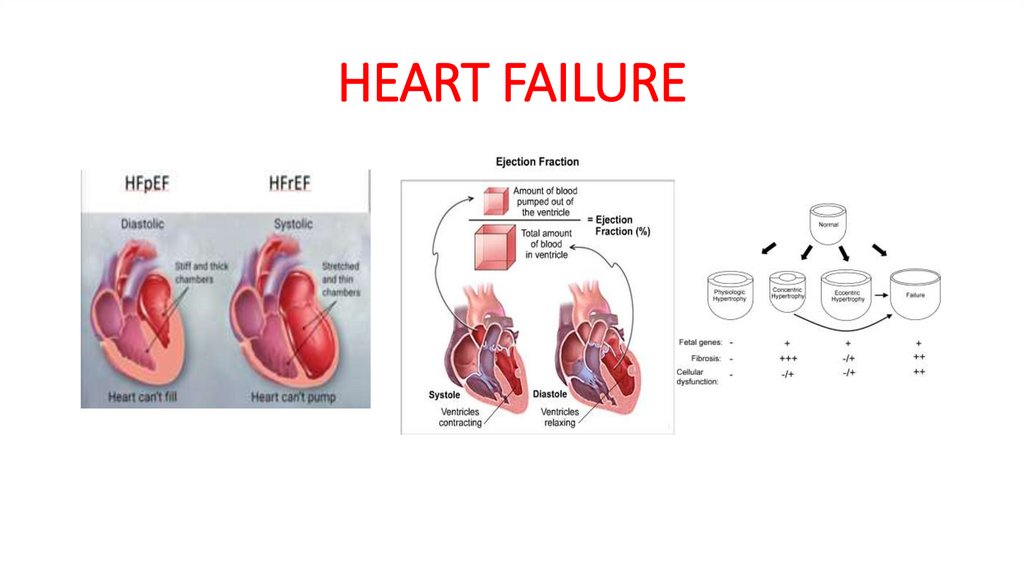
A. Background: Heart failure is a complex clinical syndromecaused by any structural or functional cardiac disorder that
impairs the ability of the ventricle to fill with or eject blood.
1- Systolic dysfunction or HF with reduced ejection fraction
(HFrEF) (decreased EF less than 40%)
4.
Characteristics:a. Impaired ventricular contraction
b. Dilated ventricle
c. Two-thirds attributable to CAD
d. One-third attributable to non-ischemic cardiomyopathy:
i. Hypertension
ii. Thyroid disease
iii. Valvular disease
iv. Cardiotoxins:
(a) Alcohol
(b) Chemotherapeutic agents
(1) Anthracyclines (2) Cyclophosphamide (3) 5-Fluorouracil
v. Tachycardia
5.
vi. Peripartum cardiomyopathy (PPCM) (occurs during the lastmonth of pregnancy or within 5 months after delivery
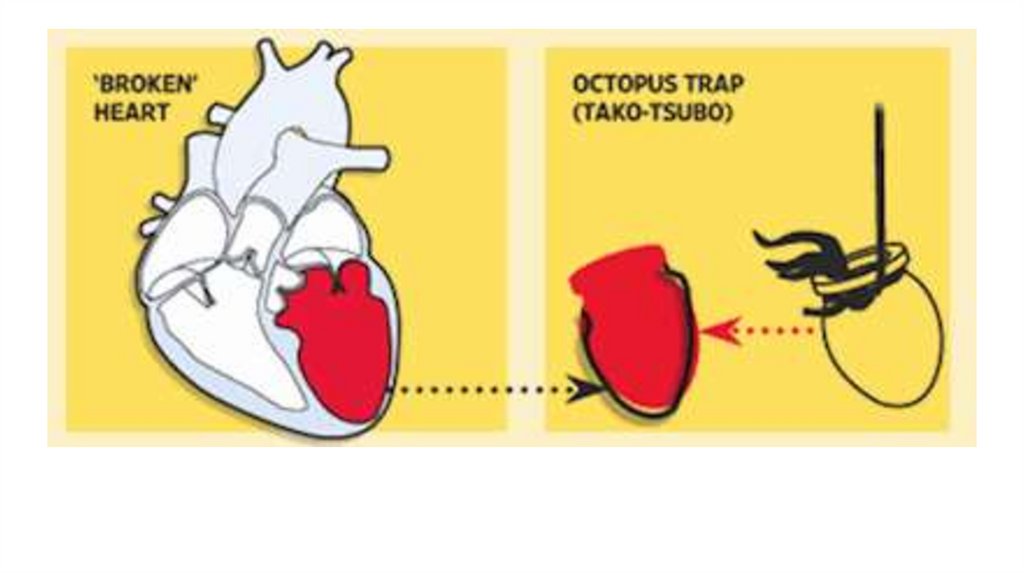
vii. Stress (Takotsubo ( كلمة يبانية معناها وعاء االخطبوطoctopus pot)),
resembling the shape of the left ventricle during systole on
imaging studies).
Although the exact etiology is still unknown, the syndrome
appears to be triggered by a significant emotional or physical
stressor. 95% of patients experiencing complete recovery within
4-8 weeks
viii. Idiopathic
6.
7.
2- Diastolic dysfunction or Heart failure with preserved EF(HFpEF) (preserved/normal EF greater than 40%)
a. Accounts for about 50% (highly variable) of patients with
HF
b. Impaired ventricular relaxation and filling
c. Normal ventricular contraction
d. Most are caused by hypertension (60-89%) and agerelated decreases in the elastic properties of the
cardiovascular system.
8.
3- HF with Mildly Reduced EF (HFmrEF)a. Defined as HF with an LVEF of 41%–49% and evidence of increased
LV filling pressures (e.g., elevated natriuretic peptides, hemodynamic
measurements)
b. Pharmacologic recommendations
i. In patients with current or previous symptoms, use of an ARNI, ACE
inhibitor, or ARB; MRA; and metoprolol succinate, carvedilol, or
bisoprolol may be considered to reduce CV mortality and HF
hospitalizations, particularly among patients with LVEF on the lower end
of the spectrum.
ii. SGLT2 inhibitors can be beneficial for decreasing CV mortality and
HF hospitalizations.
9.
4- HFimpEF: HF with improved LVEF is defined as a documented LVEFof less than 40% at baseline plus a documented LVEF improvement of
10% or greater and a second LVEF measurement of greater than 40%.
a. HFimpEF is distinct from both HFrEF and HFpEF because of distinct
biology and improved outcomes compared with the other two
causes.
b. Guideline-directed medical therapy should not be discontinued in
HFimpEF; discontinuation may cause HF recurrence.
10.
5- Primary symptomsa. Dyspnea
b. Fatigue c. Edema
d. Exercise intolerance
11.
4. Stages of HF According to ACC/AHATable. HF Failure Stages and Corresponding NYHA Functional Class
Stage (ACC/AHA)
NYHA Functional Class
A
None
At high risk of HF but without structural heart
disease or symptoms of HF
B
Structural heart disease but without signs or
I
symptoms of HF
C
Asymptomatic HF. No limitations in physical activity
caused by HF symptoms
Structural heart disease with prior or current
I
No limitations in physical activity caused by HF symptoms
symptoms of HF
II
Slight limitation of physical activity. Asymptomatic at rest
but symptoms of HF with normal level of activity
III
Marked limitations in physical activity because of HF
symptoms. Asymptomatic at rest.
IV
Symptoms of HF at rest or unable to carry out any physical
activity
D
Refractory HF requiring specialized
interventions
IV
Symptoms of HF at rest
12.
Left Ventricular Dysfunction Pathogenesis:Decreased cardiac output plus increased demands on the
heart cause increased neuro-hormonal activation which act
on heart, blood vessels and kidney leading to myocardial
fibrosis, peripheral vasoconstriction, and Na+/H2O retention
Neurohormones are angiotensin II, Norepinephrine,
Vasopressin, and aldosterone
13.
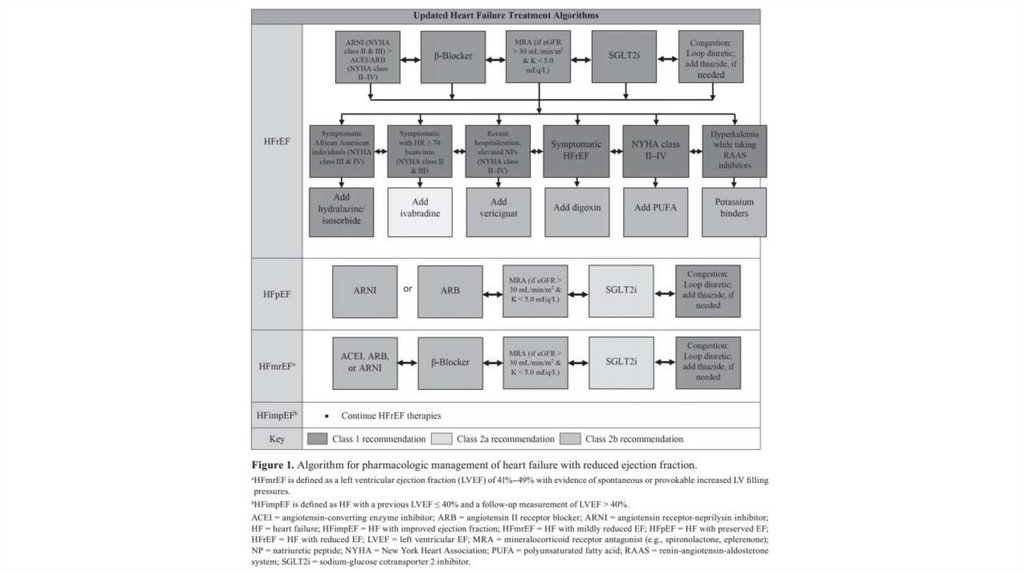
B. Pharmacologic Therapy for Systolic HF or HFrEF1. Diuretics (Management of fluid overload with (symptomatic treatment and no
mortality reduction))
a. Mechanism of action: Inhibits reabsorption of Na in the ascending limb of the loop
of Henle (loops) or in the distal tubule (thiazides)
b. place in therapy: Indicated in patients with evidence of fluid retention.
c. Dosing and administration considerations:
(a) Should generally be combined with an angiotensin-converting enzyme (ACE)
inhibitor, beta-blocker.
(b) Start with a low initial dose; may then double the dose and titrate according to the
patient’s weight and diuresis.
14.
(c) May combine with another diuretic class (e.g., thiazide diuretic)for synergy, if needed
(d) Loop diuretics are preferred because of their greater diuretic
capabilities; loop diuretics also retain efficacy with decreased renal
function.
d. Monitoring: Monitor and replace K and Mg as needed, especially
with loop diuretics (goal with cardiovascular [CV] disease is K of 4.0
mEq/L or greater and Mg of 2.0 mEq/L or greater to minimize the
risk of arrhythmias).
15.
2. Angiotensin-converting enzyme inhibitorsi. Benefits of ACE inhibitor
(a) Decreased mortality (about 25-50%)
(b) Decreased hospitalizations
(c) Symptom improvement
ii. Mechanism of action
(a) Blocks production of angiotensin II
(1) Decreases sympathetic stimulation
(2) Decreases production of aldosterone and vasopressin (aldosterone causes cardiac fibrosis)
Angiotensin II stimulates aldosterone and vasopressin secretion
(3) Decreases vasoconstriction (afterload)
(b) Increases bradykinins (decreases their metabolism) (ACEI only)
(1) Vasodilation
(2) May decrease myocardial remodeling
16.
iii. Place in therapy: Should be used in all patients with LV dysfunction (even ifasymptomatic)
iv. Dosing considerations
(a) Start low and increase (double) dose every 1–4 weeks to target dose.
(b) Patient may notice improvement in several week
Use caution if SBP is less than 90 mm Hg, SCr is greater than 3 mg/dL, or
elevated K is greater than 5.0 mEq/L or in bilateral renal artery stenosis
(absolutely contraindicated).
17.
v. Monitoring(a) Monitor SCr and K for 1–2 weeks after initiating therapy or increasing the dose,
especially in high-risk patients (preexisting hypotension, DM, K supplements, azotemia).
SCr may rise (up to a 30% increase is acceptable) because of renal efferent artery
dilation (results in a slightly decreased glomerular filtration rate). Rarely, acute renal
failure occurs, especially if the patient is intravascularly depleted. Be careful to avoid
overdiuresis.
(b) Monitor BP and symptoms of hypotension (e.g., dizziness, lightheadedness).
(c) Ninety percent of people tolerate ACE inhibitors.
(1) Angioedema (less than 1%): Can switch to angiotensin II receptor blockers (ARBs;
cross-reactivity is 2.5%) or hydralazine–isosorbide dinitrate
(2) Cough (20%): Can switch to ARBs (less than 1%)
18.
19.
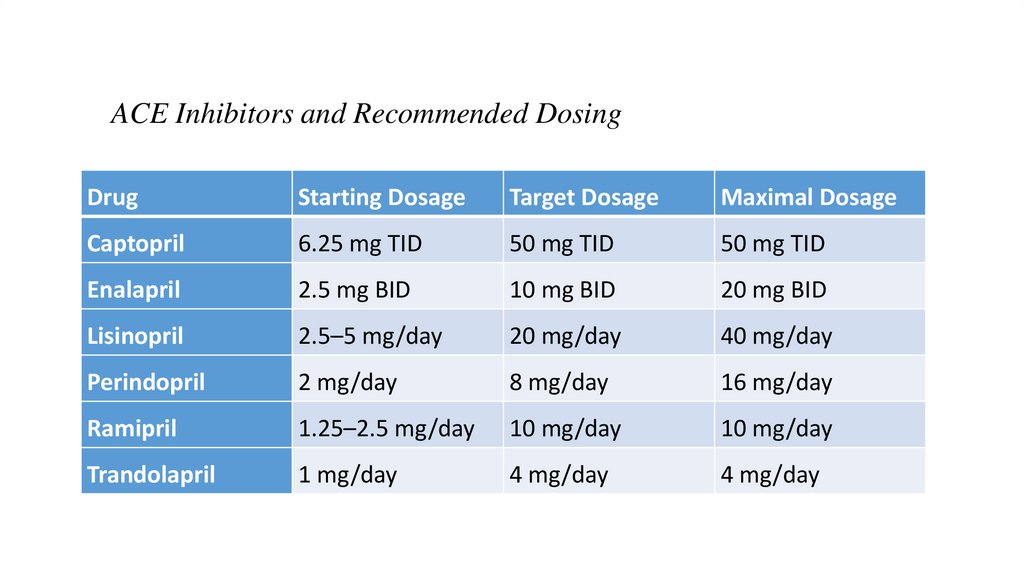
ACE Inhibitors and Recommended DosingDrug
Starting Dosage
Target Dosage
Maximal Dosage
Captopril
6.25 mg TID
50 mg TID
50 mg TID
Enalapril
2.5 mg BID
10 mg BID
20 mg BID
Lisinopril
2.5–5 mg/day
20 mg/day
40 mg/day
Perindopril
2 mg/day
8 mg/day
16 mg/day
Ramipril
1.25–2.5 mg/day
10 mg/day
10 mg/day
Trandolapril
1 mg/day
4 mg/day
4 mg/day
20.
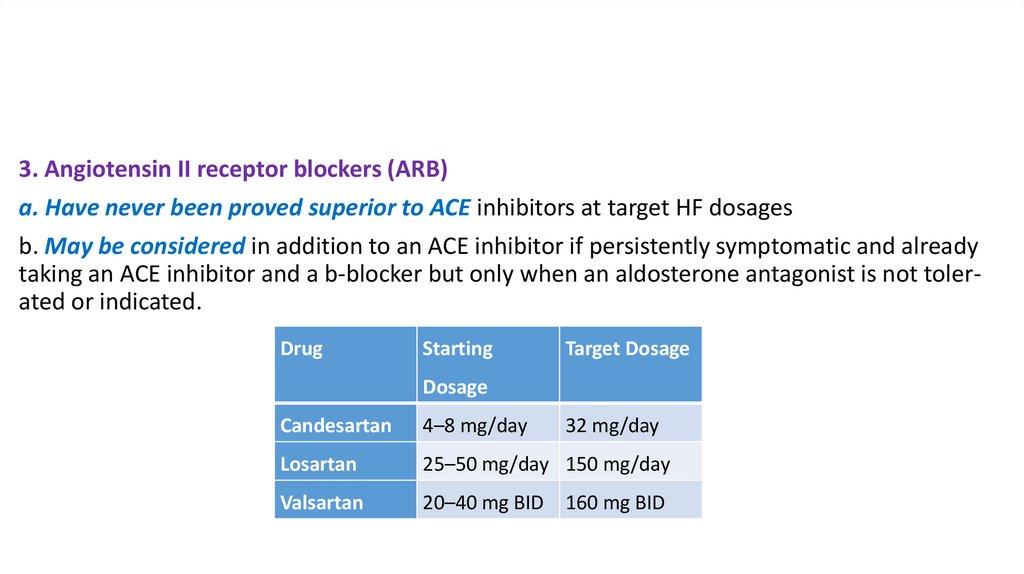
3. Angiotensin II receptor blockers (ARB)a. Have never been proved superior to ACE inhibitors at target HF dosages
b. May be considered in addition to an ACE inhibitor if persistently symptomatic and already
taking an ACE inhibitor and a b-blocker but only when an aldosterone antagonist is not tolerated or indicated.
Drug
Starting
Target Dosage
Dosage
Candesartan
4–8 mg/day
32 mg/day
Losartan
25–50 mg/day 150 mg/day
Valsartan
20–40 mg BID
160 mg BID
21.
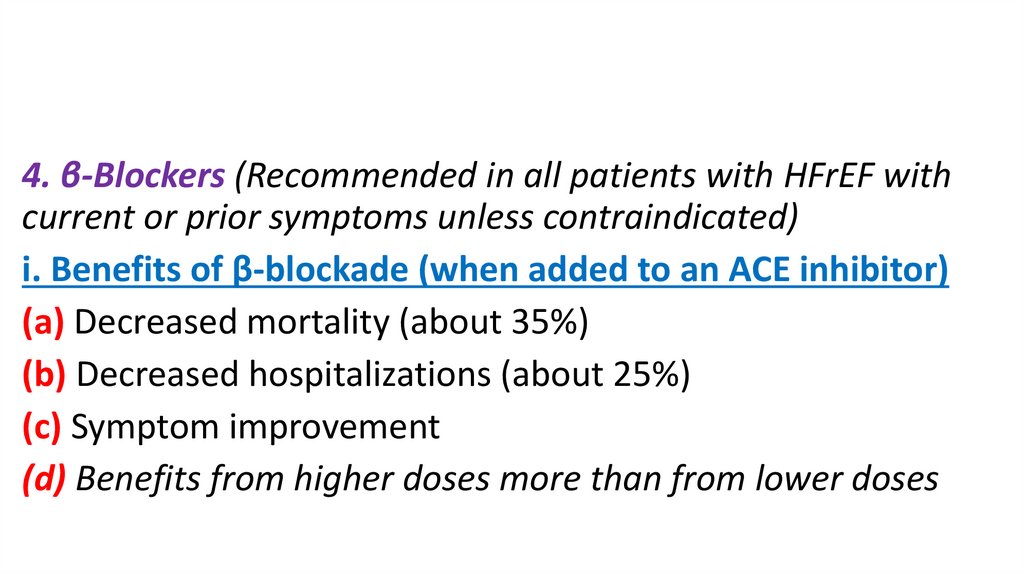
4. β-Blockers (Recommended in all patients with HFrEF withcurrent or prior symptoms unless contraindicated)
i. Benefits of β-blockade (when added to an ACE inhibitor)
(a) Decreased mortality (about 35%)
(b) Decreased hospitalizations (about 25%)
(c) Symptom improvement
(d) Benefits from higher doses more than from lower doses
22.
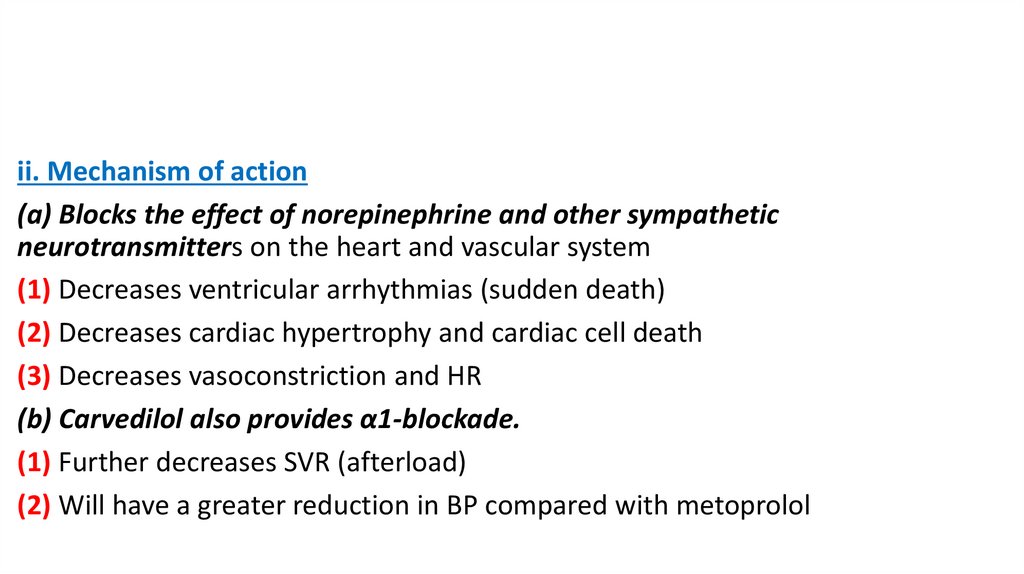
ii. Mechanism of action(a) Blocks the effect of norepinephrine and other sympathetic
neurotransmitters on the heart and vascular system
(1) Decreases ventricular arrhythmias (sudden death)
(2) Decreases cardiac hypertrophy and cardiac cell death
(3) Decreases vasoconstriction and HR
(b) Carvedilol also provides α1-blockade.
(1) Further decreases SVR (afterload)
(2) Will have a greater reduction in BP compared with metoprolol
23.
iii. Dosing considerations(a) Only bisoprolol, carvedilol, and metoprolol succinate are recommended in HFrEF
(b) Added to existing ACE inhibitor therapy (at least at a low dose) when HF symptoms are
stable and patients are euvolemic
(c) Start low and increase (double) the dose every 2 weeks (or slowly, if needed) to target
dose. Aim to achieve target dose in 8–12 weeks
(d) Avoid abrupt discontinuation; can precipitate clinical deterioration
(e) Patient may notice improvement in several months.
(f) Should be considered even in patients with reactive airway disease or asymptomatic
bradycardia
24.
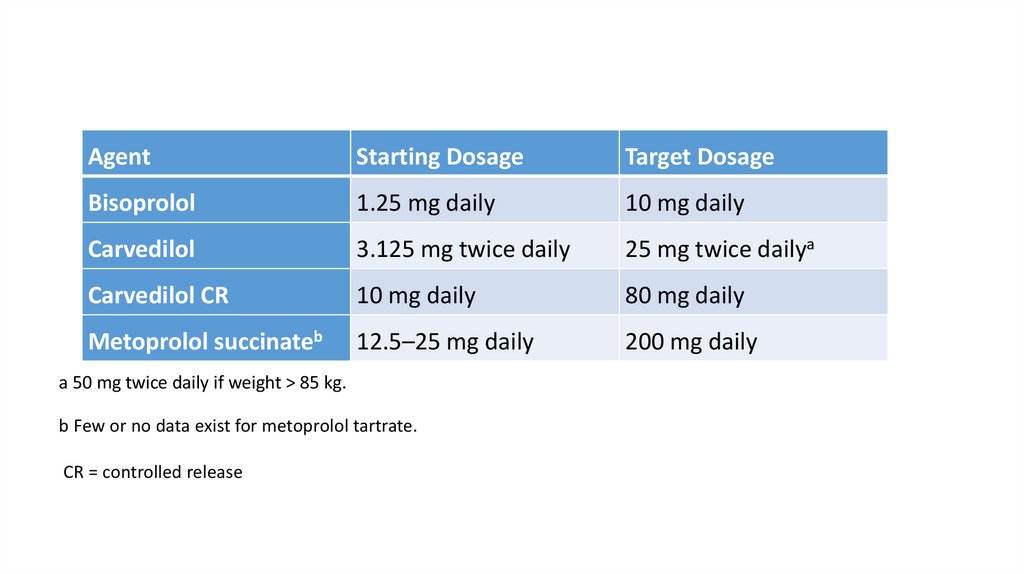
AgentStarting Dosage
Target Dosage
Bisoprolol
1.25 mg daily
10 mg daily
Carvedilol
3.125 mg twice daily
25 mg twice dailya
Carvedilol CR
10 mg daily
80 mg daily
Metoprolol succinateb
12.5–25 mg daily
200 mg daily
a 50 mg twice daily if weight > 85 kg.
b Few or no data exist for metoprolol tartrate.
CR = controlled release
25.
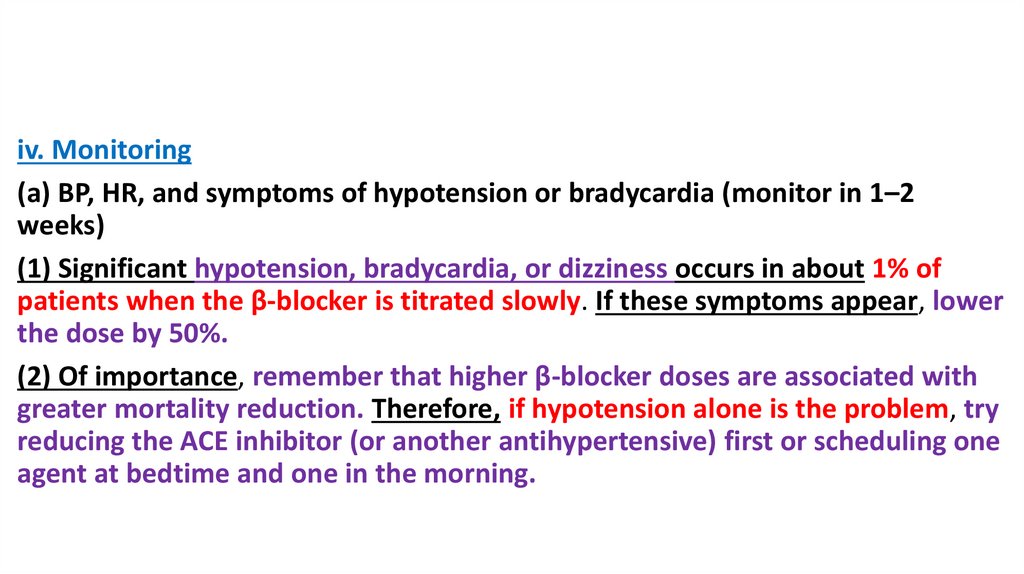
iv. Monitoring(a) BP, HR, and symptoms of hypotension or bradycardia (monitor in 1–2
weeks)
(1) Significant hypotension, bradycardia, or dizziness occurs in about 1% of
patients when the β-blocker is titrated slowly. If these symptoms appear, lower
the dose by 50%.
(2) Of importance, remember that higher β-blocker doses are associated with
greater mortality reduction. Therefore, if hypotension alone is the problem, try
reducing the ACE inhibitor (or another antihypertensive) first or scheduling one
agent at bedtime and one in the morning.
26.
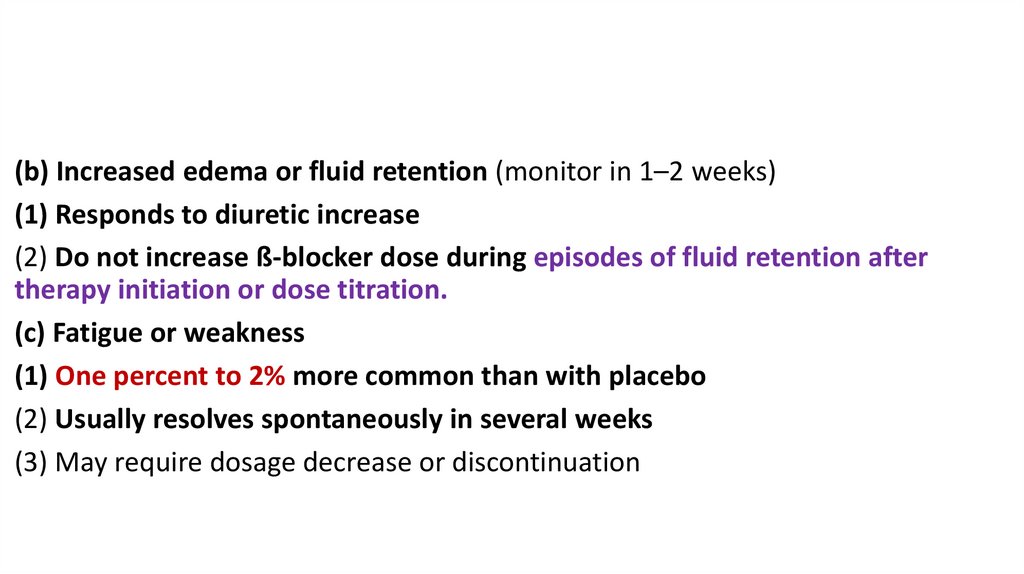
(b) Increased edema or fluid retention (monitor in 1–2 weeks)(1) Responds to diuretic increase
(2) Do not increase ß-blocker dose during episodes of fluid retention after
therapy initiation or dose titration.
(c) Fatigue or weakness
(1) One percent to 2% more common than with placebo
(2) Usually resolves spontaneously in several weeks
(3) May require dosage decrease or discontinuation
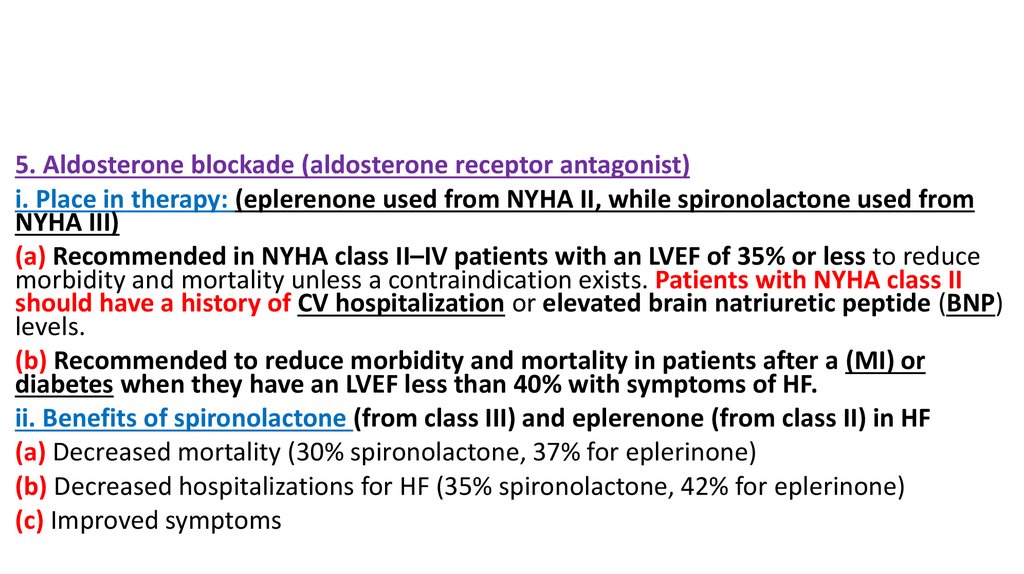
27.
5. Aldosterone blockade (aldosterone receptor antagonist)i. Place in therapy: (eplerenone used from NYHA II, while spironolactone used from
NYHA III)
(a) Recommended in NYHA class II–IV patients with an LVEF of 35% or less to reduce
morbidity and mortality unless a contraindication exists. Patients with NYHA class II
should have a history of CV hospitalization or elevated brain natriuretic peptide (BNP)
levels.
(b) Recommended to reduce morbidity and mortality in patients after a (MI) or
diabetes when they have an LVEF less than 40% with symptoms of HF.
ii. Benefits of spironolactone (from class III) and eplerenone (from class II) in HF
(a) Decreased mortality (30% spironolactone, 37% for eplerinone)
(b) Decreased hospitalizations for HF (35% spironolactone, 42% for eplerinone)
(c) Improved symptoms
28.
29.
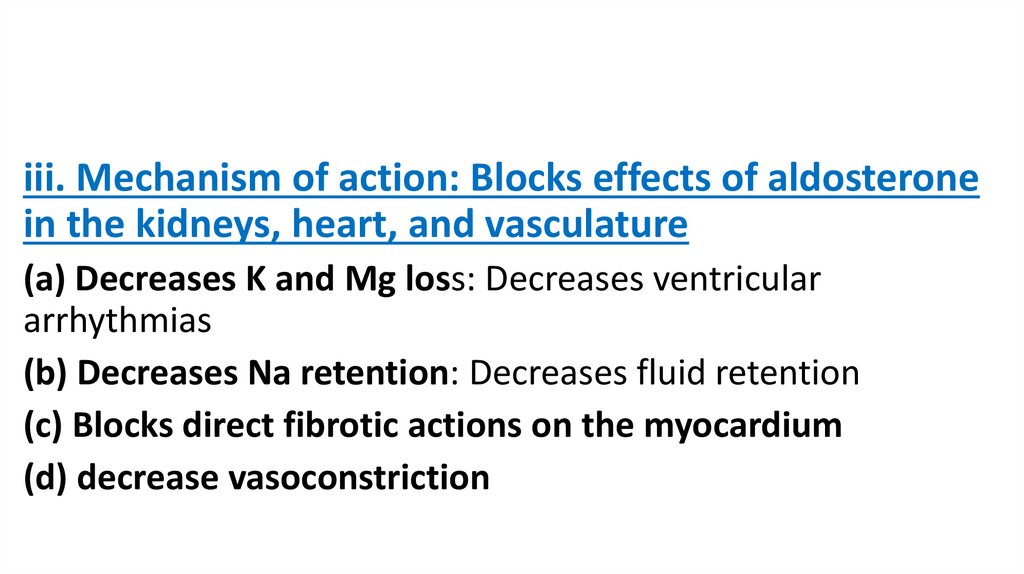
iii. Mechanism of action: Blocks effects of aldosteronein the kidneys, heart, and vasculature
(a) Decreases K and Mg loss: Decreases ventricular
arrhythmias
(b) Decreases Na retention: Decreases fluid retention
(c) Blocks direct fibrotic actions on the myocardium
(d) decrease vasoconstriction
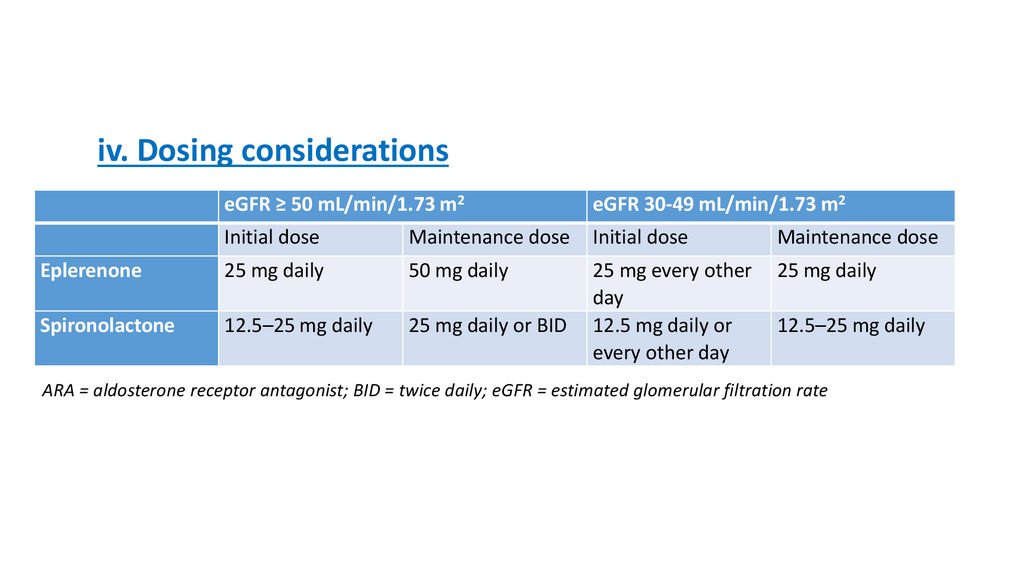
30.
iv. Dosing considerationseGFR ≥ 50 mL/min/1.73 m2
eGFR 30-49 mL/min/1.73 m2
Initial dose
Maintenance dose
Initial dose
Maintenance dose
Eplerenone
25 mg daily
50 mg daily
25 mg daily
Spironolactone
12.5–25 mg daily
25 mg daily or BID
25 mg every other
day
12.5 mg daily or
every other day
12.5–25 mg daily
ARA = aldosterone receptor antagonist; BID = twice daily; eGFR = estimated glomerular filtration rate
31.
vii. Monitoring(a) K and SCr within 2–3 days, again at 7 days after starting therapy, monthly
for first 3 months, and every 3 months thereafter.
(1) Decrease dose by 50% or discontinue if K is greater than 5.5 mEq/L.
(b) Gynecomastia
(1) For spironolactone, gynecomastia was reported at a rate of 10% in clinical
trials.
(2) Eplerenone: A selective aldosterone blocker (does not cause Gynecomastia)
32.
6. Digoxina. Benefits of digoxin
i. Improved symptoms
ii. Improved exercise tolerance
iii. Decreased hospitalizations
iv. No effect on mortality
b. Mechanism of action (in HF) by Na-K ATPase inhibition
i. Decreases central sympathetic outflow
ii. Decreases renal reabsorption of Na
iii. Minimal increase in cardiac contractility (due to Na-K ATPase inhibition)
iv. Decrease heart rate due to vagal stimulation on the heart cause reduction in A-V node
conduction
c. Place in therapy: Should be considered in patients with symptomatic LV dysfunction
despite optimal ACE inhibitor (or ARB), β-blocker, spironolactone (if appropriate), and diuretic
therapy.
33.
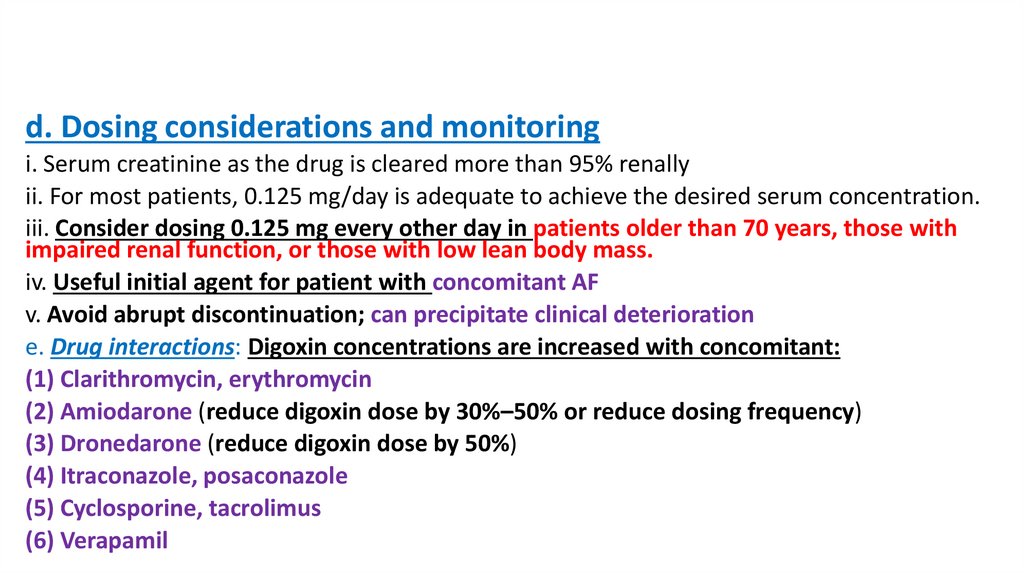
d. Dosing considerations and monitoringi. Serum creatinine as the drug is cleared more than 95% renally
ii. For most patients, 0.125 mg/day is adequate to achieve the desired serum concentration.
iii. Consider dosing 0.125 mg every other day in patients older than 70 years, those with
impaired renal function, or those with low lean body mass.
iv. Useful initial agent for patient with concomitant AF
v. Avoid abrupt discontinuation; can precipitate clinical deterioration
e. Drug interactions: Digoxin concentrations are increased with concomitant:
(1) Clarithromycin, erythromycin
(2) Amiodarone (reduce digoxin dose by 30%–50% or reduce dosing frequency)
(3) Dronedarone (reduce digoxin dose by 50%)
(4) Itraconazole, posaconazole
(5) Cyclosporine, tacrolimus
(6) Verapamil
34.
f. Monitoring: Serum concentrations should be less than 1 ng/mL,concentrations of 0.5–0.9 ng/mL are suggested.
(a) Risk of toxicity increases with age and renal dysfunction.
(b) Risk of toxicity increases in the presence of hypokalemia,
hypomagnesemia, or hypercalcemia.
(c) Signs of toxicity generally include nausea, vomiting, vision
changes (objects appear yellow or green), arrhythmia, fatigue,
headache, .
(d) SrCr should be monitored due to high dependence on renal
function for clearance.
35.
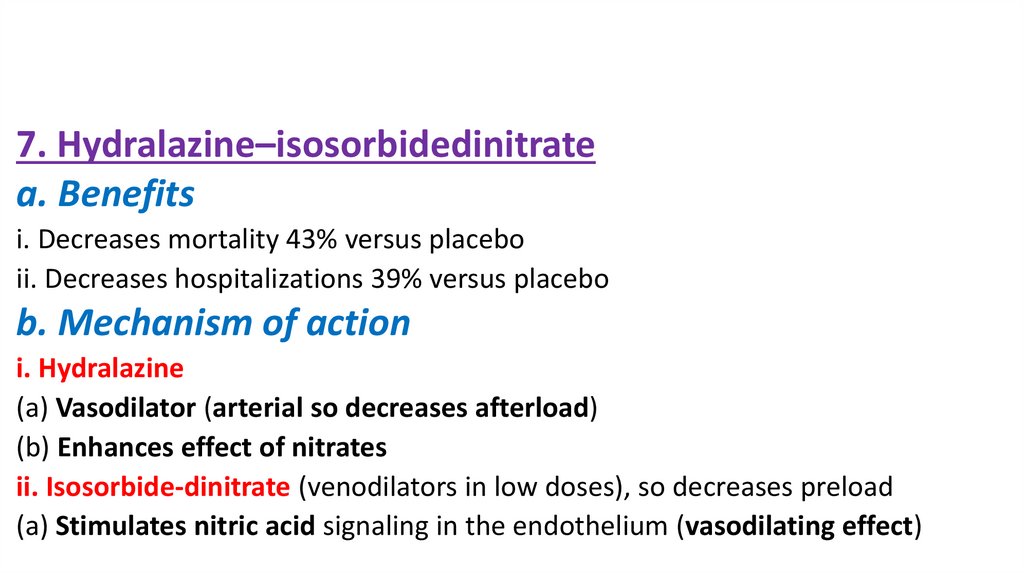
7. Hydralazine–isosorbidedinitratea. Benefits
i. Decreases mortality 43% versus placebo
ii. Decreases hospitalizations 39% versus placebo
b. Mechanism of action
i. Hydralazine
(a) Vasodilator (arterial so decreases afterload)
(b) Enhances effect of nitrates
ii. Isosorbide-dinitrate (venodilators in low doses), so decreases preload
(a) Stimulates nitric acid signaling in the endothelium (vasodilating effect)
36.
c. Place in therapyi. African Americans with NYHA class II–IV HF, already receiving an ACE inhibitor
(or ARB), β-blocker, and diuretic therapy
ii. A reasonable alternative in patients unable to take an ACE inhibitor or ARB
because of severe renal insufficiency, hyperkalemia, or angioedema
d. Adverse effects
i. Headache
ii. Hypotension
iii. Drug-induced lupus with hydralazine
37.
8. Sacubitril/valsartani. Place in therapy
(a) Novel therapy – Approved by the U.S. Food and Drug Administration in 2015.
(b) In patients with chronic symptomatic NYHA class II or III HFrEF who can tolerate an
ACE inhibitor or ARB, replacement by sacubitril/valsartan is recommended to further
reduce morbidity and mortality
(c) Stop ARB or if ACEI stop for 36 hours then start sacubitril/valsartan
ii. Benefits
Decrease mortality and hospitalization
iii. Mechanism of action
(a) Sacubitril – Prodrug metabolized to Sacubitrilat (LBQ657) which inhibits
neprilysin which destroys B-NP, so increasing levels of natriuretic peptides
38.
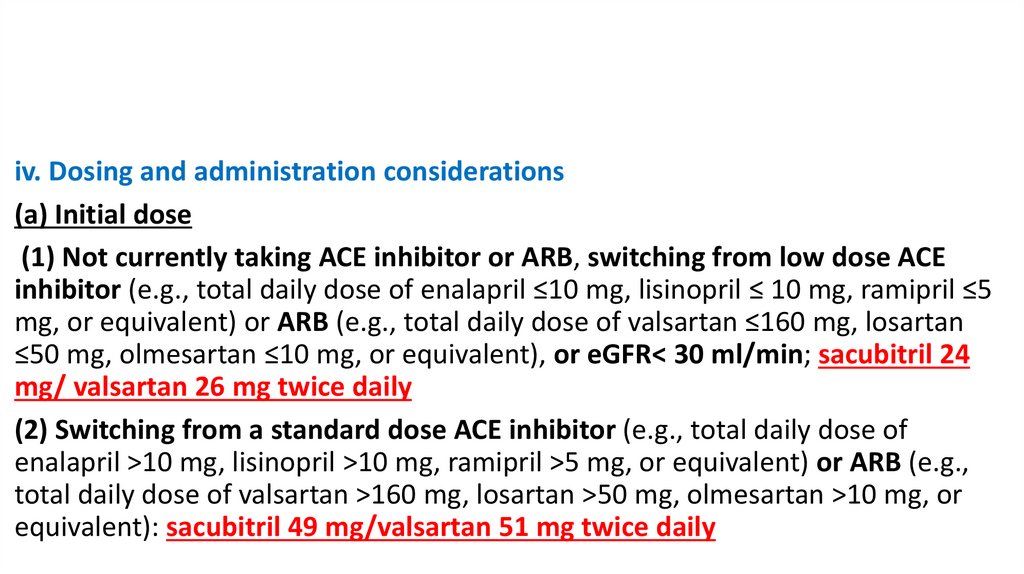
iv. Dosing and administration considerations(a) Initial dose
(1) Not currently taking ACE inhibitor or ARB, switching from low dose ACE
inhibitor (e.g., total daily dose of enalapril ≤10 mg, lisinopril ≤ 10 mg, ramipril ≤5
mg, or equivalent) or ARB (e.g., total daily dose of valsartan ≤160 mg, losartan
≤50 mg, olmesartan ≤10 mg, or equivalent), or eGFR< 30 ml/min; sacubitril 24
mg/ valsartan 26 mg twice daily
(2) Switching from a standard dose ACE inhibitor (e.g., total daily dose of
enalapril >10 mg, lisinopril >10 mg, ramipril >5 mg, or equivalent) or ARB (e.g.,
total daily dose of valsartan >160 mg, losartan >50 mg, olmesartan >10 mg, or
equivalent): sacubitril 49 mg/valsartan 51 mg twice daily
39.
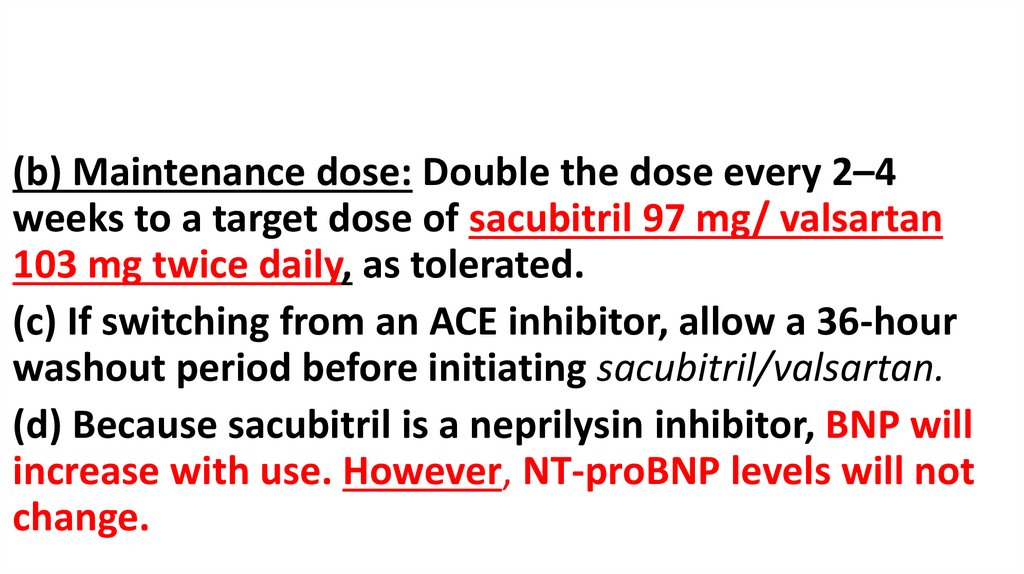
(b) Maintenance dose: Double the dose every 2–4weeks to a target dose of sacubitril 97 mg/ valsartan
103 mg twice daily, as tolerated.
(c) If switching from an ACE inhibitor, allow a 36-hour
washout period before initiating sacubitril/valsartan.
(d) Because sacubitril is a neprilysin inhibitor, BNP will
increase with use. However, NT-proBNP levels will not
change.
40.
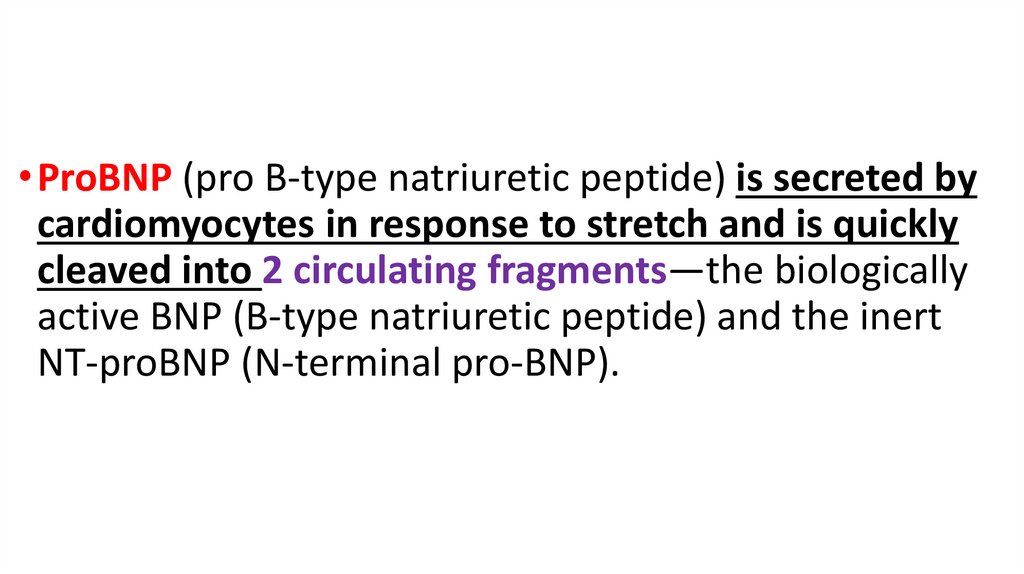
• ProBNP (pro B-type natriuretic peptide) is secreted bycardiomyocytes in response to stretch and is quickly
cleaved into 2 circulating fragments—the biologically
active BNP (B-type natriuretic peptide) and the inert
NT-proBNP (N-terminal pro-BNP).
41.
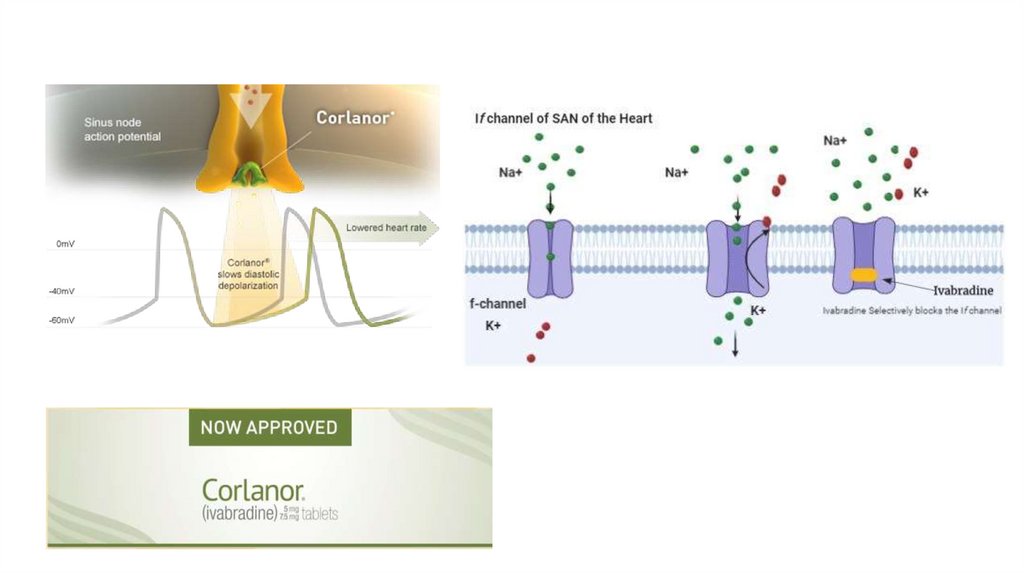
9. Ivabradinei. Place in therapy
(a) Novel therapy – Approved by the FDA in 2015
(b) Ivabradine can be beneficial to reduce HF hospitalizations for patients with
symptomatic (NYHA class II and III), chronic HFrEF (LVEF of 35% or less) who are
receiving evidence-based therapies, including a β-blocker at maximum
tolerated dose, and who are in sinus rhythm (SR) with a heart rate of 70 beats/
minute or greater at rest.
ii. Mechanism of action – Selectively inhibits the If (funny channel) current in
the sinoatrial node, providing heart rate reduction
42.
43.
iii. Dosing and administration considerations(a) Given the well-proven mortality benefits of β-blocker therapy, it is important to
initiate and titrate these agents to target doses, as tolerated, before assessing the
resting heart rate for consideration of ivabradine initiation.
(b) Initial dosing: 5 mg twice daily
(c) After 2 weeks, adjust dose according to heart rate:
(1) Resting heart rate greater than 60 beats/minute: Increase dose by 2.5 mg twice
daily.
(2) Resting heart rate 50–60 beats/minute: Continue current dose.
(3) Resting heart rate less than 50 beats/minute or signs/symptoms of bradycardia:
Decrease dose by 2.5 mg twice daily.
(d) Maximum dose: 7.5 mg twice daily
44.
iv. Monitoring –(a) Assess heart rate after 2 weeks of therapy initiation or modification
and periodically thereafter.
(b) Phosphenes (3%): transient rings or spots of light in the visual field
v. Contraindications:
i.
ADHF,
ii. BP <90/50 mm Hg,
iii. resting HR< 60 beats/min,
iv. sinoatrial block,
v. concomitant use with strong CYP3A4 inhibitors
45.
Other medication therapiesi. Omega-3 fatty acids: Reasonable adjunctive therapy in NYHA class II–IV
symptoms and HFrEF or HFpEF (class IIa recommendation)
ii. Antiarrhythmics: Amiodarone and dofetilide are the preferred anti-arrhythmics
that should be used in HFrEF for patients with arrhythmias given neutral effects on
mortality.
iii. Calcium channel blockers: Non-dihydropyridine calcium channel blockers with
negative inotropic effects may be harmful and should be avoided. Use of
amlodipine can be considered for HTN or ischemic heart disease management in
HF patients because of its neutral effects on morbidity and mortality
iv. Dapagliflozin: May be used as an add-on therapy in persistently symptomatic
HFrEF with elevated N-terminal pro-brain natriuretic peptide levels in eligible
patients with or without diabetes
46.
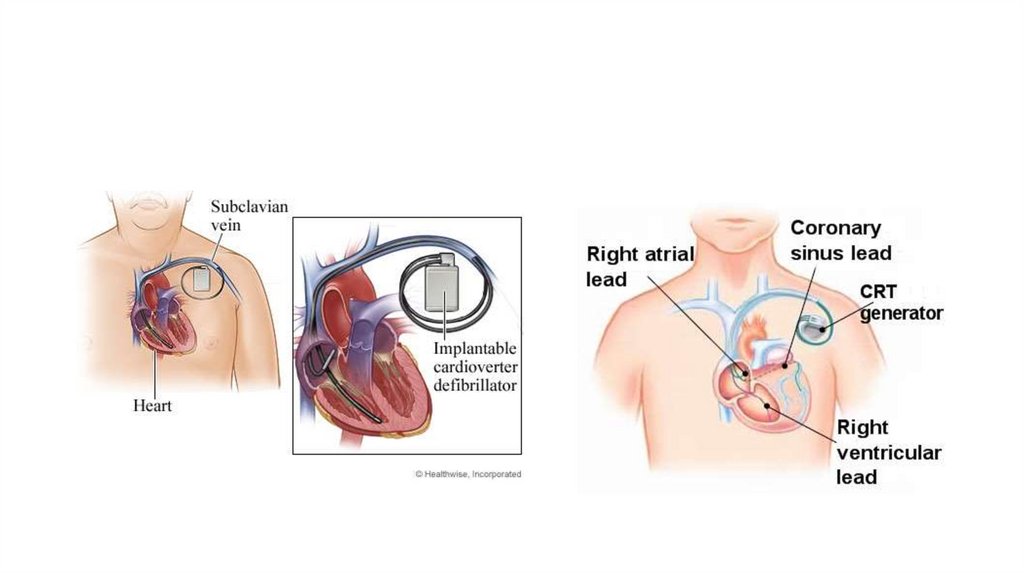
Device therapyi. Implantable cardioverter defibrillator (ICD): Recommended for primary prevention
of sudden cardiac death in ischemic and non-ischemic patients
(a) Qualifying criteria include:
A. Patients 40 days post-MI, LVEF of 35% or less, or NYHA class II or III symptoms
on chronic optimal medical therapy. Life expectancy should be greater than 1
year (class I indication).
B. 40 days post-MI, LVEF of 30% or less, and NYHA class I symptoms on chronic
optimal medical therapy. Life expectancy should be greater than 1 year (class I
indication).
ii. Chronic resynchronization therapy: Recommended for those with an LVEF of 35%
or less, in SR, and a left bundle branch block with a QRS of 150 milliseconds or
greater on optimal medical therapy with NYHA class II–III symptoms or NYHA class
IV with ambulation
47.
48.
49.
50.
C. Drugs to Avoid or Use with Caution1. Drugs that promote sodium and water retention
a. Nonsteroidal anti-inflammatory drugs (NSAIDs, including selective
cyclooxygenase-2 inhibitors)
i. Promote Na and water retention
ii. Blunt diuretic response
iii. Increase morbidity and mortality
b. Corticosteroids
c. Minoxidil
d. Thiazolidinediones
51.
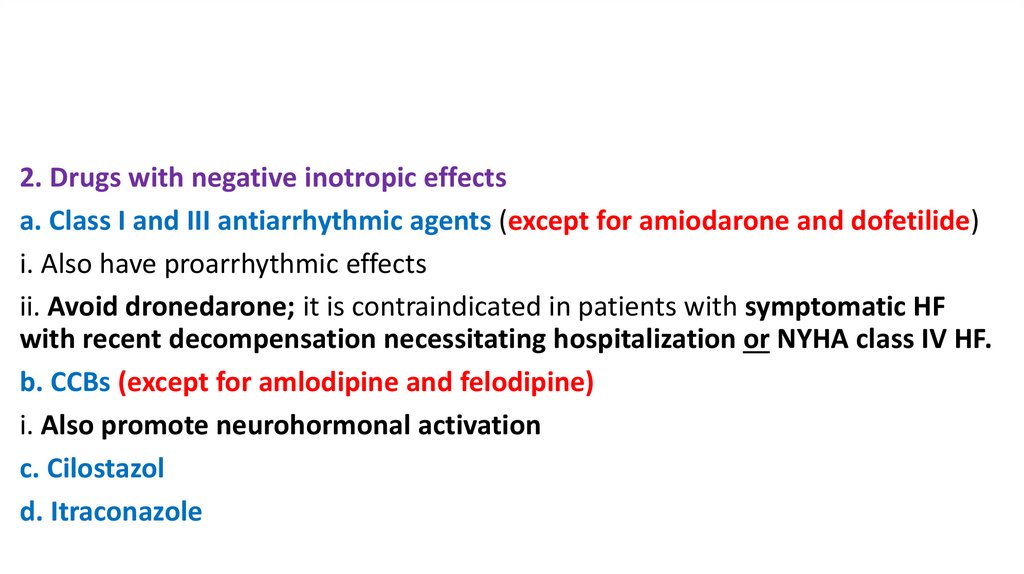
2. Drugs with negative inotropic effectsa. Class I and III antiarrhythmic agents (except for amiodarone and dofetilide)
i. Also have proarrhythmic effects
ii. Avoid dronedarone; it is contraindicated in patients with symptomatic HF
with recent decompensation necessitating hospitalization or NYHA class IV HF.
b. CCBs (except for amlodipine and felodipine)
i. Also promote neurohormonal activation
c. Cilostazol
d. Itraconazole
52.
3. Othera. Metformin: Increased risk of lactic acidosis (black box warning)
b. Amphetamines (e.g., methylphenidate)
i. α- and β-agonist activity
ii. Cause tachycardia
iii. Proarrhythmic effects
c. Pregabalin
i. Inhibits calcium channels
ii. Lower-extremity edema, HF exacerbation
53.
D. Pharmacologic Therapy for Diastolic Dysfunction (no mortality reduction)For diastolic dysfunction you have to decrease blood pressure, edema, and heart
rate
a. SBP and diastolic blood pressure (DBP) should be well controlled. HTN impairs
myocardial relaxation and promotes cardiac hypertrophy. Goal SBP less than 130 mm
Hg
b. Diuretics should be used for symptom relief in volume overload.
c. The use of β-blockers, ACE inhibitors, and ARBs in patients with HTN is reasonable
to control BP
d. Control of HR improves symptoms of HF.
i. Can use β-blockers or non-DHP CCBs
ii. DHP CCBs may cause reflex tachycardia, potentiating diastolic dysfunction.
54.
Angiotensin-converting enzyme inhibitors or ARBsa. Reduction in hospitalizations
b. Improvement in symptoms
c. Improvement in exercice tolerance
β-Blockers, verapamil, and diltiazem: Benefits are targeted symptom relief
(superior over ACEI when HR is high).
55.
56.
Acute decompensated heart failureA. Diagnosis
1. Must include a detailed history and physical examination
57.
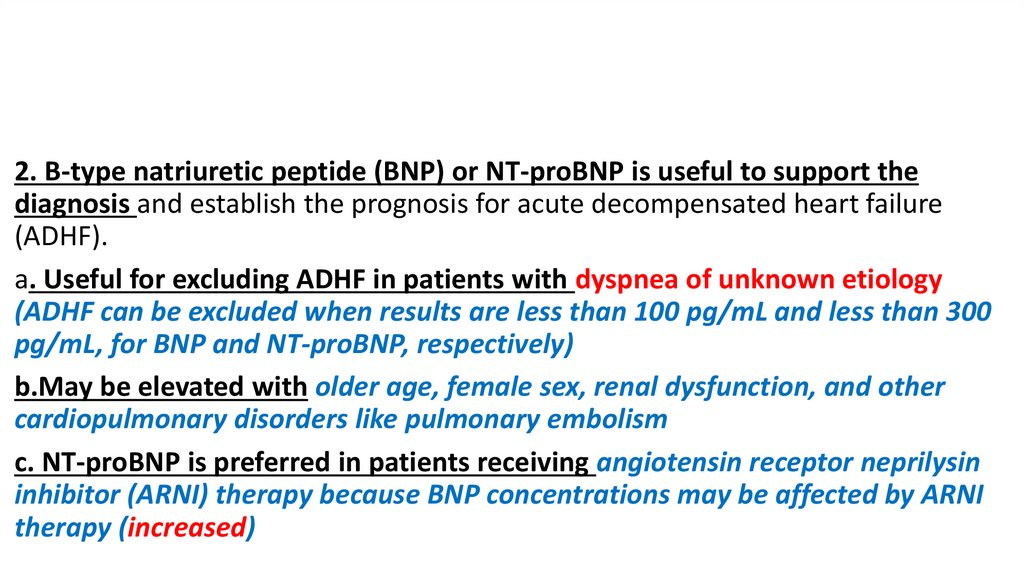
2. B-type natriuretic peptide (BNP) or NT-proBNP is useful to support thediagnosis and establish the prognosis for acute decompensated heart failure
(ADHF).
a. Useful for excluding ADHF in patients with dyspnea of unknown etiology
(ADHF can be excluded when results are less than 100 pg/mL and less than 300
pg/mL, for BNP and NT-proBNP, respectively)
b.May be elevated with older age, female sex, renal dysfunction, and other
cardiopulmonary disorders like pulmonary embolism
c. NT-proBNP is preferred in patients receiving angiotensin receptor neprilysin
inhibitor (ARNI) therapy because BNP concentrations may be affected by ARNI
therapy (increased)
58.
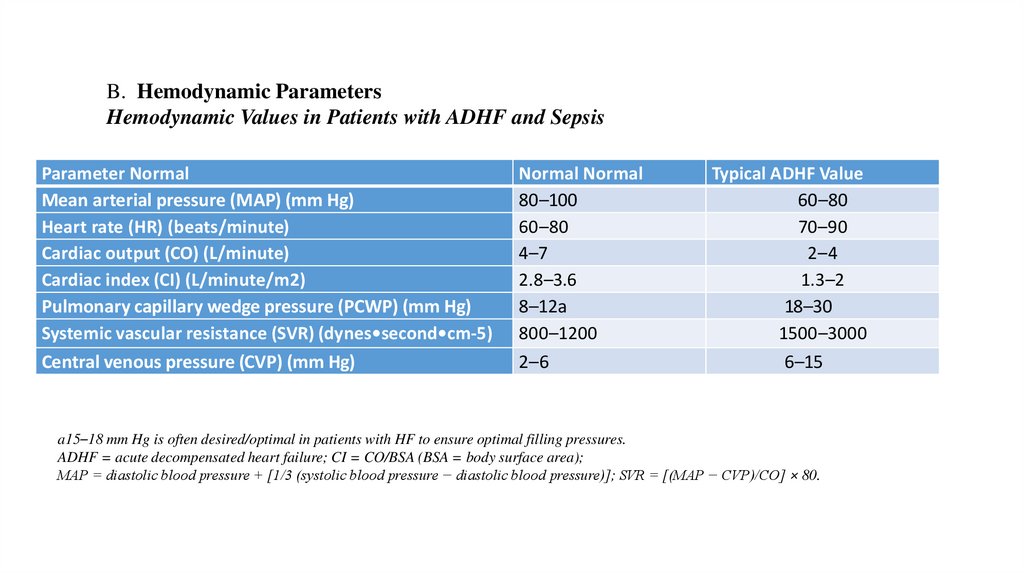
B. Hemodynamic ParametersHemodynamic Values in Patients with ADHF and Sepsis
Parameter Normal
Mean arterial pressure (MAP) (mm Hg)
Heart rate (HR) (beats/minute)
Cardiac output (CO) (L/minute)
Cardiac index (CI) (L/minute/m2)
Pulmonary capillary wedge pressure (PCWP) (mm Hg)
Systemic vascular resistance (SVR) (dynes•second•cm-5)
Central venous pressure (CVP) (mm Hg)
Normal Normal
80–100
60–80
4–7
2.8–3.6
8–12a
800–1200
2–6
Typical ADHF Value
60–80
70–90
2–4
1.3–2
18–30
1500–3000
6–15
a15–18 mm Hg is often desired/optimal in patients with HF to ensure optimal filling pressures.
ADHF = acute decompensated heart failure; CI = CO/BSA (BSA = body surface area);
MAP = diastolic blood pressure + [1/3 (systolic blood pressure − diastolic blood pressure)]; SVR = [(MAP − CVP)/CO] × 80.
59.
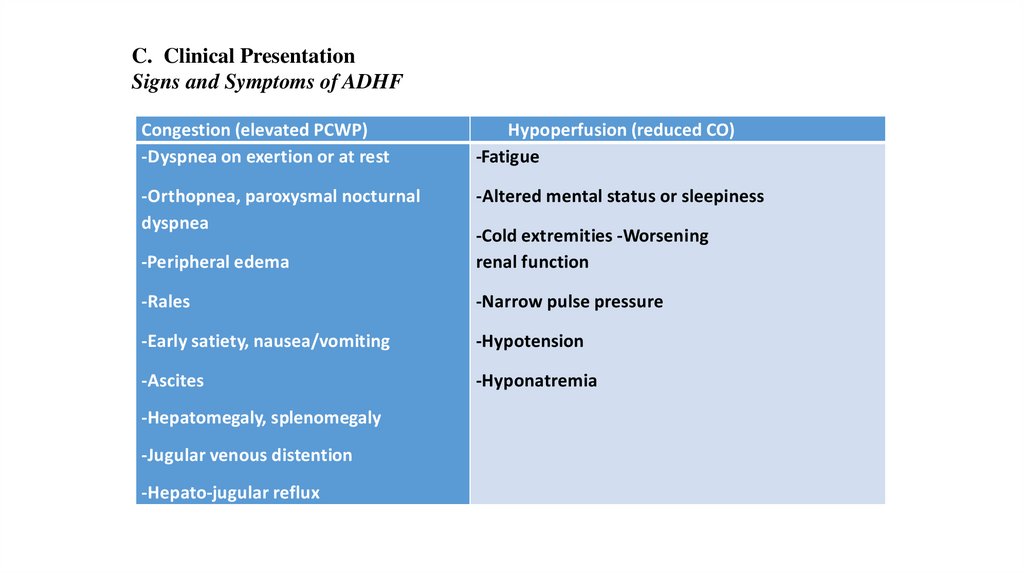
C. Clinical PresentationSigns and Symptoms of ADHF
Congestion (elevated PCWP)
-Dyspnea on exertion or at rest
Hypoperfusion (reduced CO)
-Fatigue
-Orthopnea, paroxysmal nocturnal
dyspnea
-Altered mental status or sleepiness
-Peripheral edema
-Cold extremities -Worsening
renal function
-Rales
-Narrow pulse pressure
-Early satiety, nausea/vomiting
-Hypotension
-Ascites
-Hyponatremia
-Hepatomegaly, splenomegaly
-Jugular venous distention
-Hepato-jugular reflux
60.
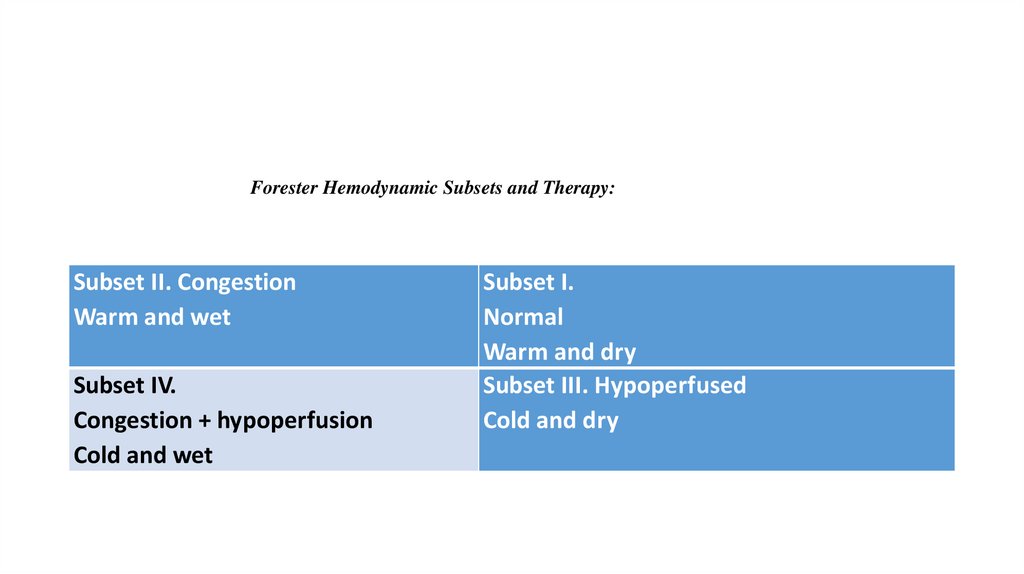
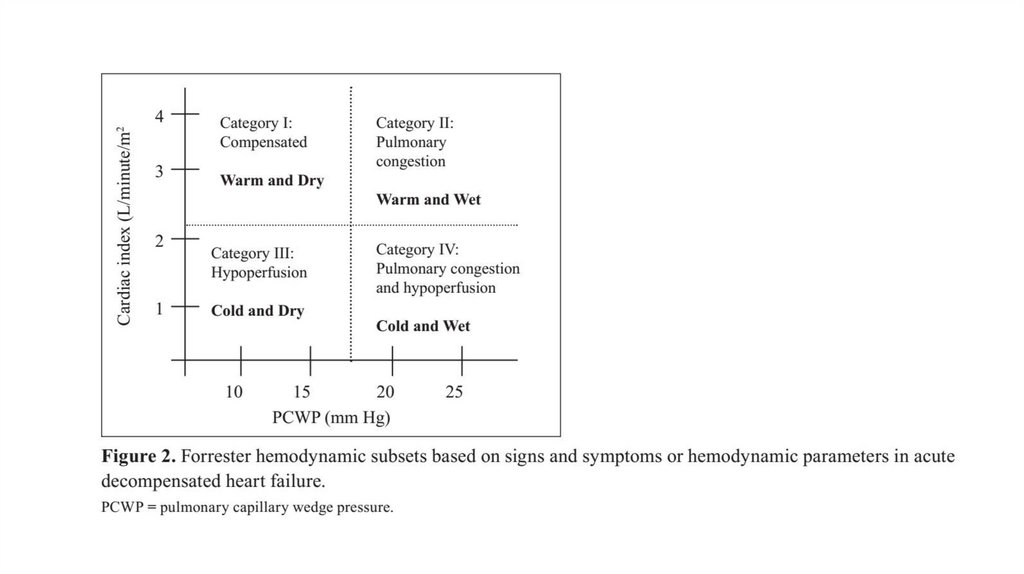
Forester Hemodynamic Subsets and Therapy:Subset II. Congestion
Warm and wet
Subset IV.
Congestion + hypoperfusion
Cold and wet
Subset I.
Normal
Warm and dry
Subset III. Hypoperfused
Cold and dry
61.
62.
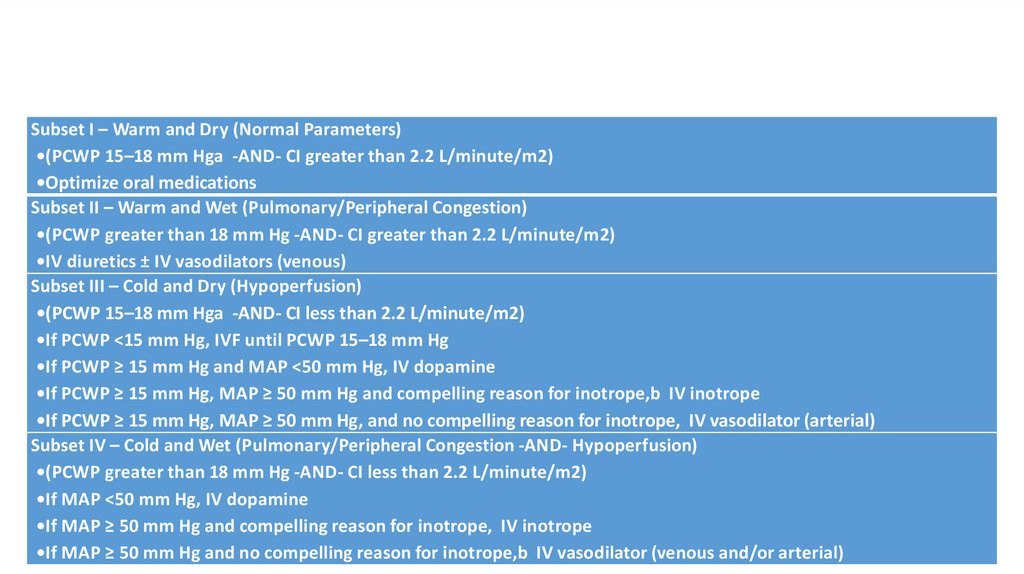
Subset I – Warm and Dry (Normal Parameters)•(PCWP 15–18 mm Hga -AND- CI greater than 2.2 L/minute/m2)
•Optimize oral medications
Subset II – Warm and Wet (Pulmonary/Peripheral Congestion)
•(PCWP greater than 18 mm Hg -AND- CI greater than 2.2 L/minute/m2)
•IV diuretics ± IV vasodilators (venous)
Subset III – Cold and Dry (Hypoperfusion)
•(PCWP 15–18 mm Hga -AND- CI less than 2.2 L/minute/m2)
•If PCWP <15 mm Hg, IVF until PCWP 15–18 mm Hg
•If PCWP ≥ 15 mm Hg and MAP <50 mm Hg, IV dopamine
•If PCWP ≥ 15 mm Hg, MAP ≥ 50 mm Hg and compelling reason for inotrope,b IV inotrope
•If PCWP ≥ 15 mm Hg, MAP ≥ 50 mm Hg, and no compelling reason for inotrope, IV vasodilator (arterial)
Subset IV – Cold and Wet (Pulmonary/Peripheral Congestion -AND- Hypoperfusion)
•(PCWP greater than 18 mm Hg -AND- CI less than 2.2 L/minute/m2)
•If MAP <50 mm Hg, IV dopamine
•If MAP ≥ 50 mm Hg and compelling reason for inotrope, IV inotrope
•If MAP ≥ 50 mm Hg and no compelling reason for inotrope,b IV vasodilator (venous and/or arterial)
63.
Overview of ADHF Guideline RecommendationsDiuretic therapy
i. Recommended as intravenous loop diuretics for patients with fluid overload. Change to oral route on day before discharge, if possible.
ii. When response to diuretics is minimal, the following options should be considered:
(a) Fluid and sodium restriction
(b) Initiation of increased doses and IV doses
(c) Addition of a second diuretic with a different mechanism of action (metolazone, hydrochlorothiazide, chlorothiazide)
(d) Continuous infusion of loop diuretic
(e) Ultrafiltration
Inotropic therapy
i. May be considered to relieve symptoms and improve end-organ function in patients with reduced left ventricular ejection fraction and diminished peripheral perfusion or end-organ
dysfunction (low output syndrome), particularly if
(a) Marginal systolic blood pressure (<90 mm Hg)
(b) Symptomatic hypotension despite adequate filling pressure
(c) Worsening renal function
(d) No response to or intolerance of intravenous vasodilators
Vasodilator therapy
i. May be considered in addition to intravenous loop diuretics to rapidly improve symptoms in patients with acute pulmonary edema or severe hypertension (if symptomatic hypotension
absents)
ii. May be considered in patients with persistent symptoms despite aggressive diuretics and oral drug therapy
iii. When adjunctive therapy is necessary in addition to loop diuretics, intravenous vasodilators should be considered over inotropic drugs
64.
D. Vasodilator Therapy1. Used (with diuretics) primarily to manage pulmonary
congestion or wet (subset II or IV) HF
a. Use is limited to relief of dyspnea in those with intact
blood pressure.
b. No data to suggest intravenous vasodilators improve
outcomes
2. When adequate blood pressure is maintained, use in
preference to inotropic therapy.
65.
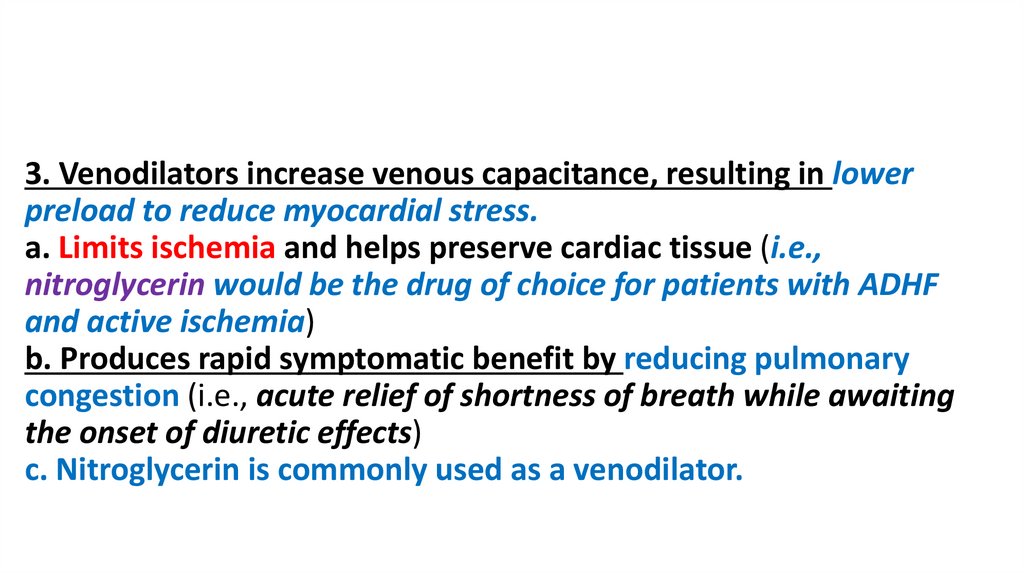
3. Venodilators increase venous capacitance, resulting in lowerpreload to reduce myocardial stress.
a. Limits ischemia and helps preserve cardiac tissue (i.e.,
nitroglycerin would be the drug of choice for patients with ADHF
and active ischemia)
b. Produces rapid symptomatic benefit by reducing pulmonary
congestion (i.e., acute relief of shortness of breath while awaiting
the onset of diuretic effects)
c. Nitroglycerin is commonly used as a venodilator.
66.
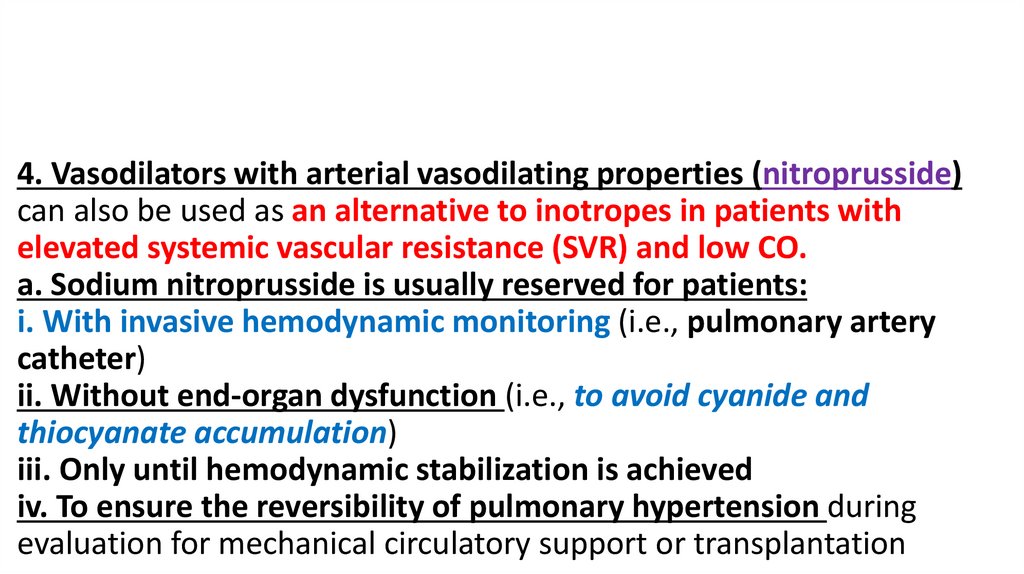
4. Vasodilators with arterial vasodilating properties (nitroprusside)can also be used as an alternative to inotropes in patients with
elevated systemic vascular resistance (SVR) and low CO.
a. Sodium nitroprusside is usually reserved for patients:
i. With invasive hemodynamic monitoring (i.e., pulmonary artery
catheter)
ii. Without end-organ dysfunction (i.e., to avoid cyanide and
thiocyanate accumulation)
iii. Only until hemodynamic stabilization is achieved
iv. To ensure the reversibility of pulmonary hypertension during
evaluation for mechanical circulatory support or transplantation
67.
b. Nesiritide was discontinued by the manufacturer inFebruary 2018.
5. Vasodilators should be avoided in patients with
symptomatic hypotension (i.e., SBP less than 90 mm
Hg).
6. Frequent blood pressure monitoring is necessary.
68.
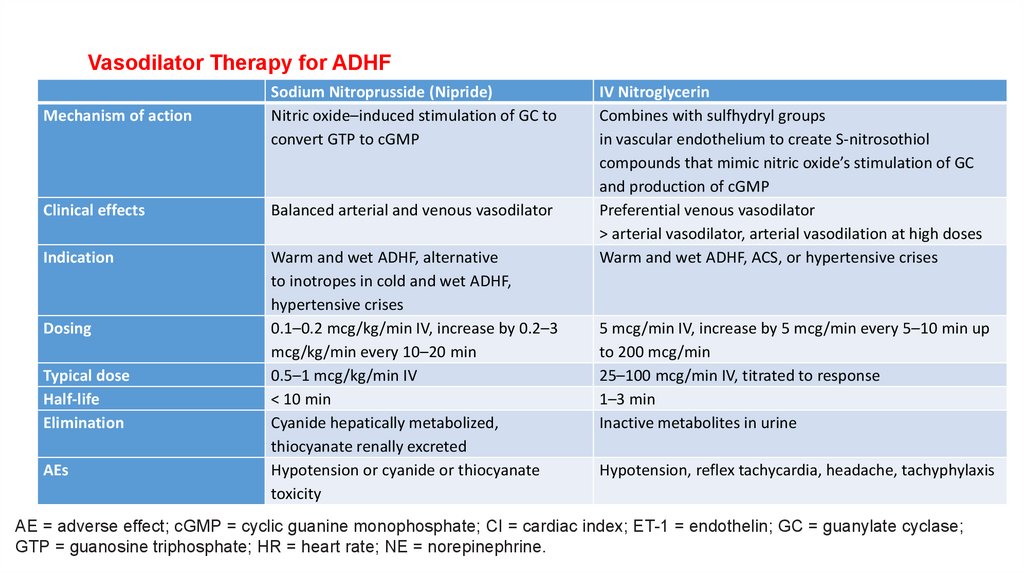
Vasodilator Therapy for ADHFMechanism of action
Sodium Nitroprusside (Nipride)
Nitric oxide–induced stimulation of GC to
convert GTP to cGMP
Clinical effects
Balanced arterial and venous vasodilator
Indication
Warm and wet ADHF, alternative
to inotropes in cold and wet ADHF,
hypertensive crises
0.1–0.2 mcg/kg/min IV, increase by 0.2–3
mcg/kg/min every 10–20 min
0.5–1 mcg/kg/min IV
< 10 min
Cyanide hepatically metabolized,
thiocyanate renally excreted
Hypotension or cyanide or thiocyanate
toxicity
Dosing
Typical dose
Half-life
Elimination
AEs
IV Nitroglycerin
Combines with sulfhydryl groups
in vascular endothelium to create S-nitrosothiol
compounds that mimic nitric oxide’s stimulation of GC
and production of cGMP
Preferential venous vasodilator
> arterial vasodilator, arterial vasodilation at high doses
Warm and wet ADHF, ACS, or hypertensive crises
5 mcg/min IV, increase by 5 mcg/min every 5–10 min up
to 200 mcg/min
25–100 mcg/min IV, titrated to response
1–3 min
Inactive metabolites in urine
Hypotension, reflex tachycardia, headache, tachyphylaxis
AE = adverse effect; cGMP = cyclic guanine monophosphate; CI = cardiac index; ET-1 = endothelin; GC = guanylate cyclase;
GTP = guanosine triphosphate; HR = heart rate; NE = norepinephrine.
69.
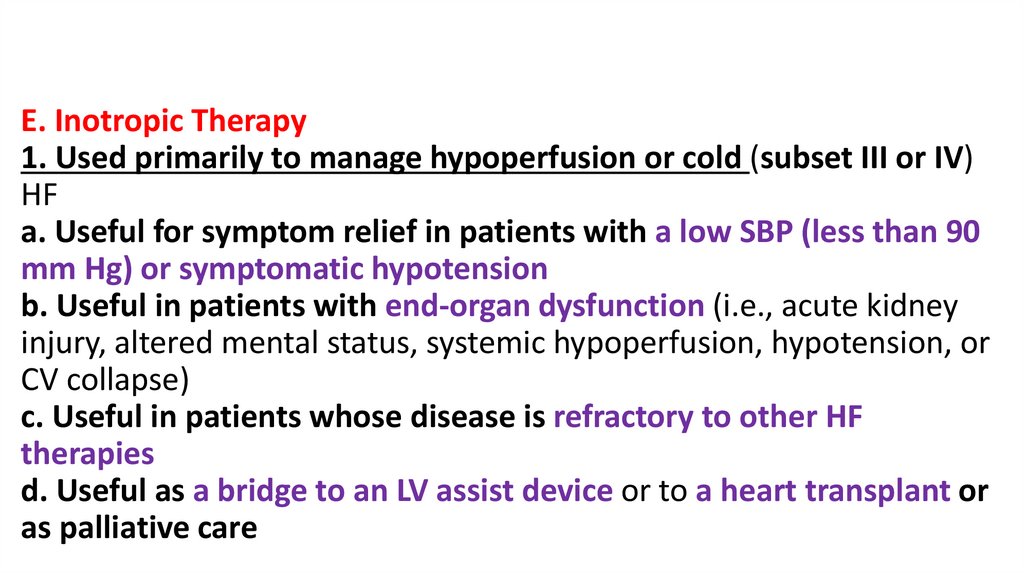
E. Inotropic Therapy1. Used primarily to manage hypoperfusion or cold (subset III or IV)
HF
a. Useful for symptom relief in patients with a low SBP (less than 90
mm Hg) or symptomatic hypotension
b. Useful in patients with end-organ dysfunction (i.e., acute kidney
injury, altered mental status, systemic hypoperfusion, hypotension, or
CV collapse)
c. Useful in patients whose disease is refractory to other HF
therapies
d. Useful as a bridge to an LV assist device or to a heart transplant or
as palliative care
70.
2. Given the risk of sequelae, it is reasonable toconsider vasodilators before inotropes.
a. Both milrinone and dobutamine are proarrhythmic.
b. Inotropes increase mortality compared with
vasodilator therapy.
3. Monitor continuously for arrhythmias.
71.
4. Differences in the pharmacologic effects of dobutamine and milrinone mayconfer advantages and disadvantages, but the choice of inotropic therapy is
very individualized.
a. Milrinone may be favored:
i. To avoid tapering or discontinuing home β-blocker
ii. When pulmonary artery pressures are high
b. Dobutamine may be favored in:
i. Severe hypotension
ii. Bradycardia
iii. Thrombocytopenia
iv. Severe renal impairment
72.
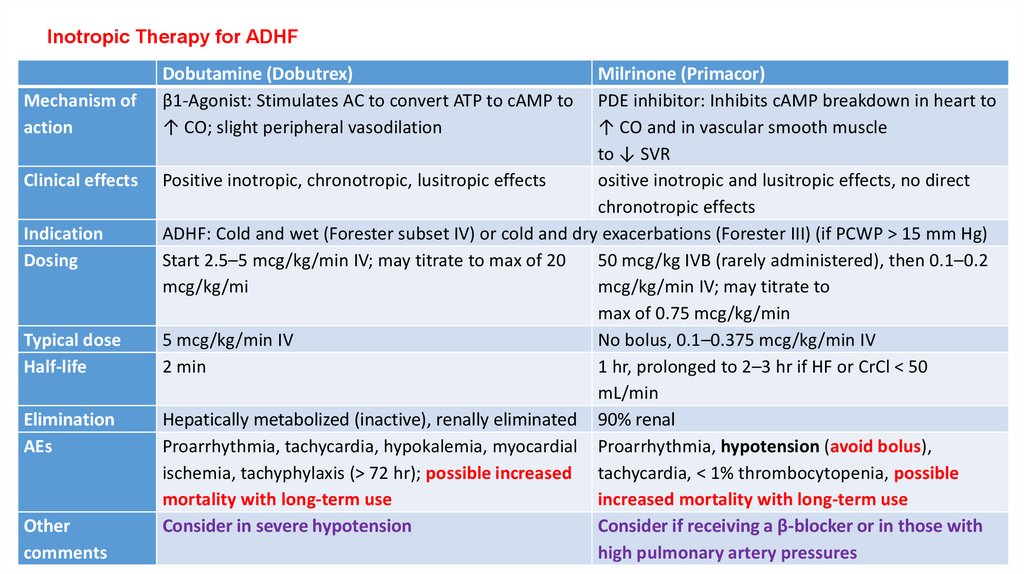
Inotropic Therapy for ADHFMechanism of
action
Clinical effects
Indication
Dosing
Typical dose
Half-life
Elimination
AEs
Other
comments
Dobutamine (Dobutrex)
β1-Agonist: Stimulates AC to convert ATP to cAMP to
↑ CO; slight peripheral vasodilation
Milrinone (Primacor)
PDE inhibitor: Inhibits cAMP breakdown in heart to
↑ CO and in vascular smooth muscle
to ↓ SVR
Positive inotropic, chronotropic, lusitropic effects
ositive inotropic and lusitropic effects, no direct
chronotropic effects
ADHF: Cold and wet (Forester subset IV) or cold and dry exacerbations (Forester III) (if PCWP > 15 mm Hg)
Start 2.5–5 mcg/kg/min IV; may titrate to max of 20
50 mcg/kg IVB (rarely administered), then 0.1–0.2
mcg/kg/mi
mcg/kg/min IV; may titrate to
max of 0.75 mcg/kg/min
5 mcg/kg/min IV
No bolus, 0.1–0.375 mcg/kg/min IV
2 min
1 hr, prolonged to 2–3 hr if HF or CrCl < 50
mL/min
Hepatically metabolized (inactive), renally eliminated 90% renal
Proarrhythmia, tachycardia, hypokalemia, myocardial Proarrhythmia, hypotension (avoid bolus),
ischemia, tachyphylaxis (> 72 hr); possible increased tachycardia, < 1% thrombocytopenia, possible
mortality with long-term use
increased mortality with long-term use
Consider in severe hypotension
Consider if receiving a β-blocker or in those with
high pulmonary artery pressures
73.
F. Vasopressin Antagonists1. Use is limited because of their significant cost and their limited
effects on meaningful long-term ADHF outcomes.
a. Should be viewed as “add on” therapy to aggressive diuresis and
not as initial or adjunctive therapy for fluid removal
b. Strict free water restriction is guideline recommended (expert
opinion).
2. Tolvaptan is FDA approved for clinically significant hyponatremia
associated with HF.
74.
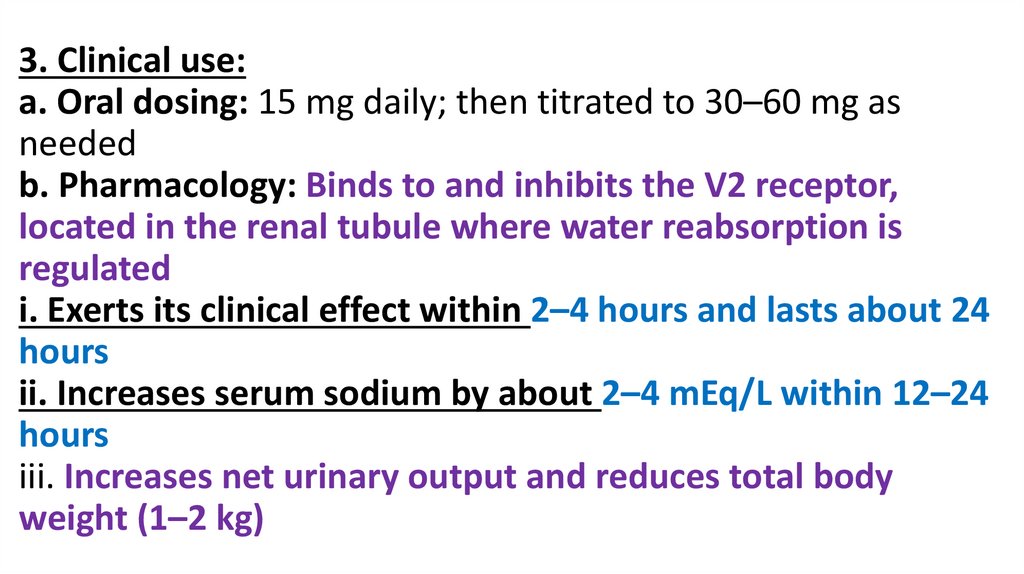
3. Clinical use:a. Oral dosing: 15 mg daily; then titrated to 30–60 mg as
needed
b. Pharmacology: Binds to and inhibits the V2 receptor,
located in the renal tubule where water reabsorption is
regulated
i. Exerts its clinical effect within 2–4 hours and lasts about 24
hours
ii. Increases serum sodium by about 2–4 mEq/L within 12–24
hours
iii. Increases net urinary output and reduces total body
weight (1–2 kg)
75.
c. Initiate only in the hospital setting to allow monitoring ofvolume status and serum sodium concentrations.
d. Contraindicated with CYP3A4 inhibitors (tolvaptan is a
substrate of 3A4) and in those with an eCrCl less than 10
mL/minute
e. The FDA warns against use beyond 30 days (i.e.,
hepatotoxicity).
i. Hyponatremia redevelops after therapy cessation.





























 Медицина
Медицина