Похожие презентации:
Applied analytical chemistry
1.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
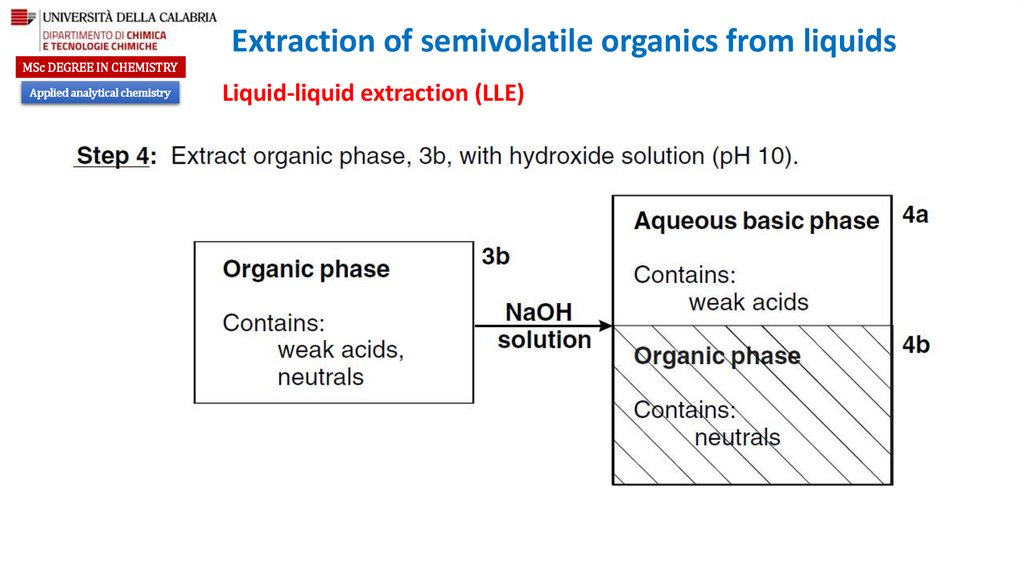
Liquid-liquid extraction (LLE)
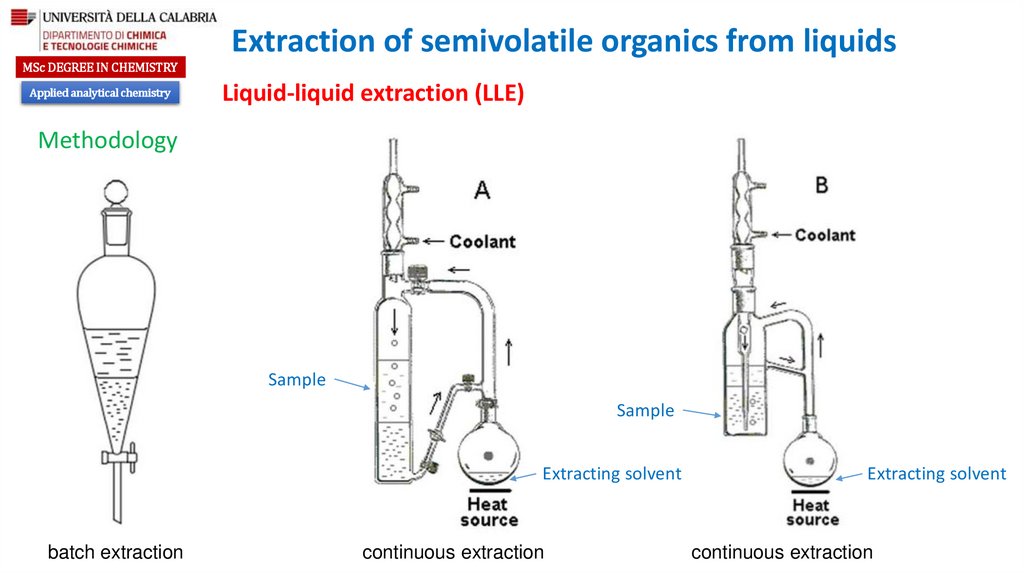
Methodology
Sample
Sample
Extracting solvent
batch extraction
continuous extraction
Extracting solvent
continuous extraction
2.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
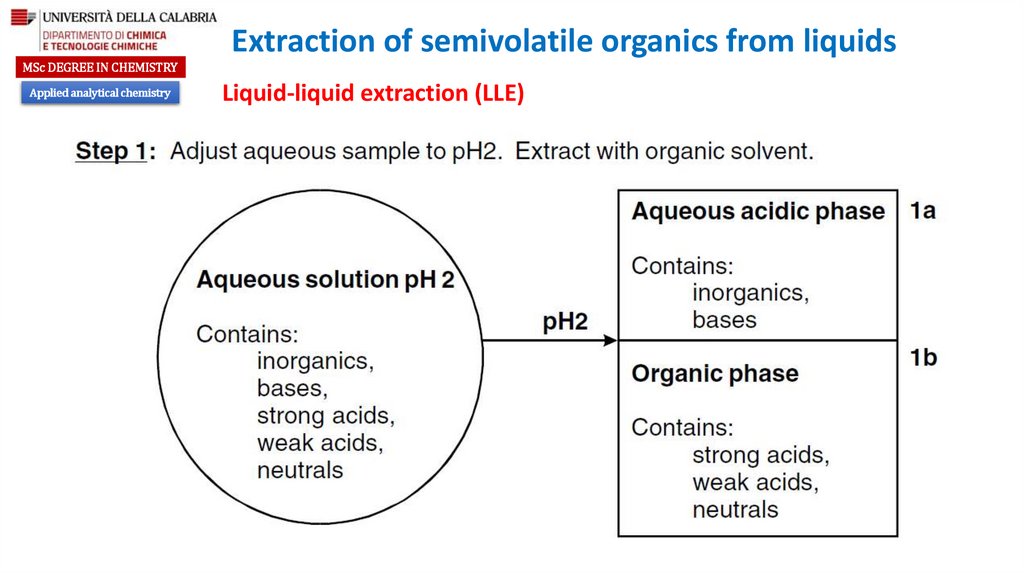
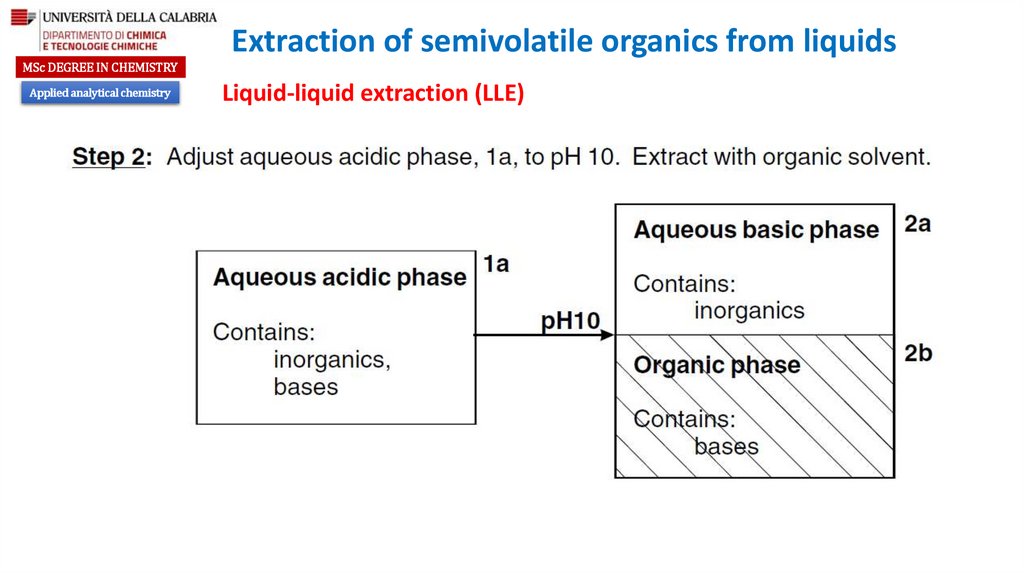
Liquid-liquid extraction (LLE)
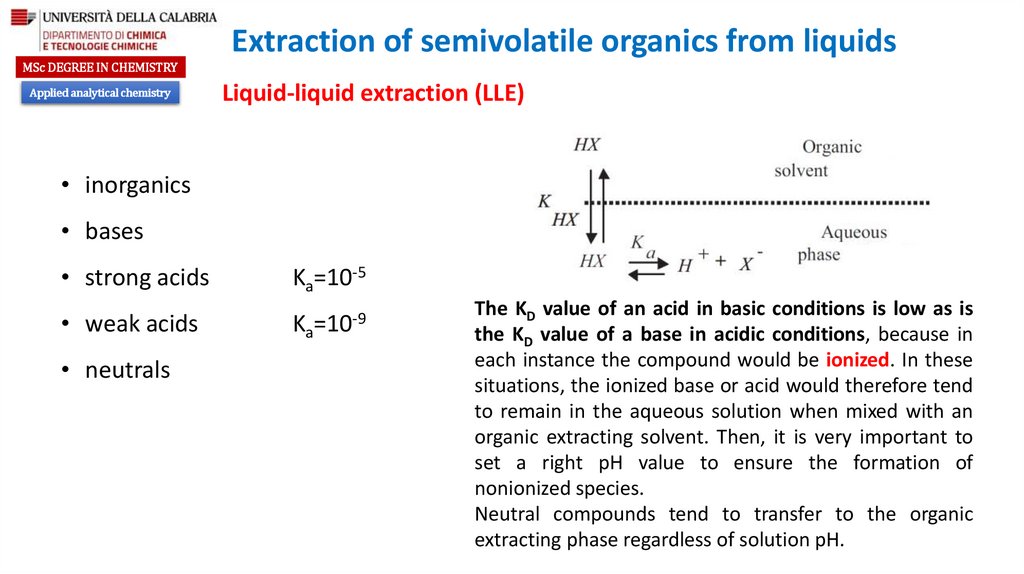
• inorganics
• bases
• strong acids
Ka=10-5
• weak acids
Ka=10-9
• neutrals
The KD value of an acid in basic conditions is low as is
the KD value of a base in acidic conditions, because in
each instance the compound would be ionized. In these
situations, the ionized base or acid would therefore tend
to remain in the aqueous solution when mixed with an
organic extracting solvent. Then, it is very important to
set a right pH value to ensure the formation of
nonionized species.
Neutral compounds tend to transfer to the organic
extracting phase regardless of solution pH.
3.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Liquid-liquid extraction (LLE)
4.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Liquid-liquid extraction (LLE)
5.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Liquid-liquid extraction (LLE)
washing
back-extraction
retro-extraction
6.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Liquid-liquid extraction (LLE)
7.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Liquid-liquid extraction (LLE)
8.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Liquid-liquid extraction (LLE)
9.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
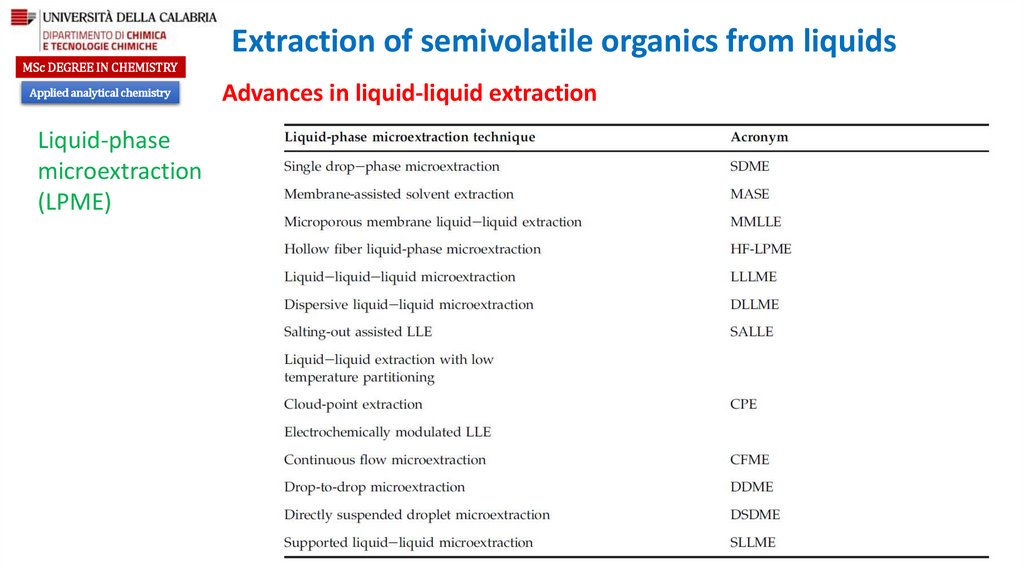
Liquid-phase
microextraction
(LPME)
Advances in liquid-liquid extraction
10.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
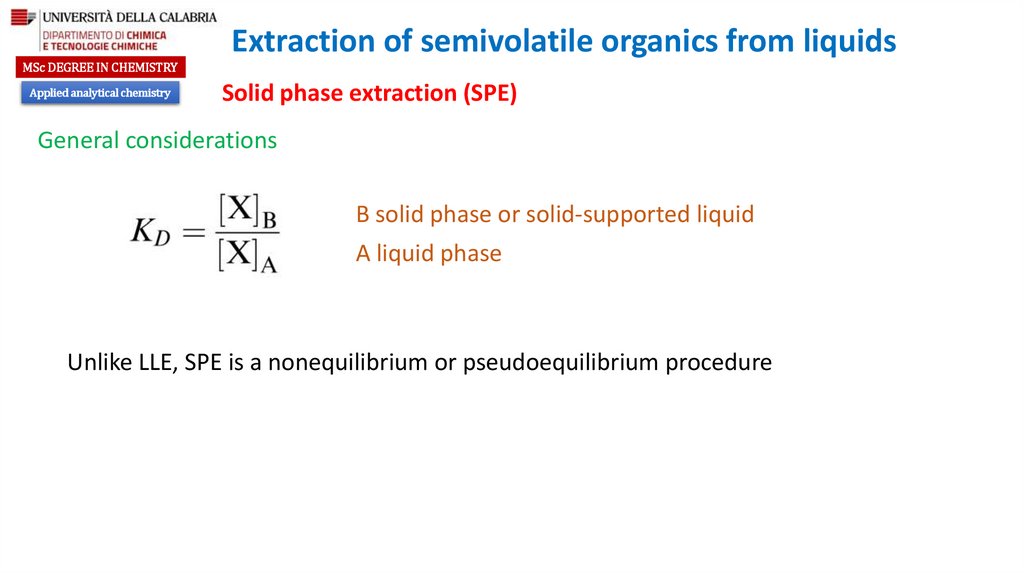
Solid phase extraction (SPE)
General considerations
B solid phase or solid-supported liquid
A liquid phase
Unlike LLE, SPE is a nonequilibrium or pseudoequilibrium procedure
11.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
General considerations
absorption meaning into a three-dimensional matrix
adsorption as meaning onto a two-dimensional surface
1. Through absorption, the analyte may interact with the
sorbent by penetrating its three-dimensional structure,
similar to water being absorbed by a sponge.
2. The analyte may interact two-dimensionally with the
sorbent surface through adsorption due to intermolecular
forces such as van der Waals or dipole–dipole interactions.
3. If the compound is ionogenic (or ionizable) in aqueous
solution, there may be an electrostatic attraction between
the analyte and charged sites on the sorbent surface.
4. The analyte and the sorbent may be chemically reactive
toward each other such that the analyte becomes
covalently bonded to the solid-phase sorbent.
12.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
General considerations
Porous sorbents vary in pore size,
shape, and tortuosity and are
characterized by properties such as
particle diameter
pore diameter
pore volume
surface areas
particle-size distribution
13.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
General considerations
Sorption tendency is dependent on the characters of the sorbent, the liquid sample
matrix, and the analyte. Much of the driving force for extracting semivolatile organics
from liquids onto a solid sorbent results from the favorable energy gains achieved when
transferring between phases.
For some of the sorbents discussed in this part of course, the solid-supported liquid
sorbent phase performing the extraction may appear to the naked eye to be a solid when
it is actually a liquid.
14.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
General considerations
When the liquid extracting phase simply coats a solid support instead of bonding to the
surface, it continues to behave primarily like a liquid; that is, the solid-supported liquid
phase still has three-dimensional freedom of motion and the sorptive behavior observed
is dominated by absorption processes.
When the liquid extracting phase is covalently bonded to the surface, it no longer acts
primarily like a bulk liquid, since there is freedom of movement in two dimensions only;
translational and rotational movement are restricted; and retention on this type of phase
can no longer be described solely by absorption processes. Retention on a liquid phase
covalently bonded to a porous solid support does not result from a pure absorption or a
pure adsorption mechanism.
15.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
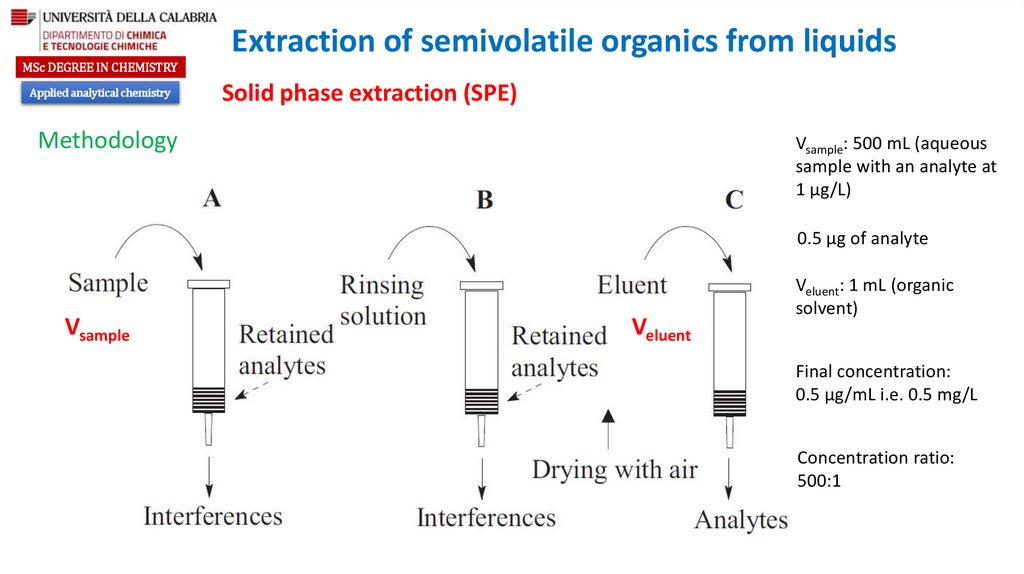
Methodology
Vsample: 500 mL (aqueous
sample with an analyte at
1 µg/L)
0.5 µg of analyte
Vsample
Veluent
Veluent: 1 mL (organic
solvent)
Final concentration:
0.5 µg/mL i.e. 0.5 mg/L
Concentration ratio:
500:1
16.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Methodology
Solid phase extraction (SPE)
17.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Methodology
Solid phase extraction (SPE)
18.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Methodology
Solid-phase extraction refers to the nonequilibrium, exhaustive removal of chemical
constituents from a flowing liquid sample via retention on a contained solid sorbent and
subsequent recovery of selected constituents by elution from the sorbent.
Mathematically, a strong affinity equates to a large KD value in equation of analyte
distribution because the concentration in the sorbent extracting phase, [X]B, is large
relative to those in the sample extracted. For this reason, SPE is sometimes referred to as
digital chromatography, indicating the all-or-nothing extremes in the sorptive nature of
these sorbents, caused by the strong attraction for the analyte by the sorbent. SPE drives
liquid chromatographic mechanisms to their extreme, such that KD approaches infinity,
representing total accumulation of the analyte during retention, and KD approaches zero
during subsequent elution or release of the analyte.
19.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Methodology
The analyte molecules that exist in true homogeneous solution in the sample are not
filtered; they become associated with the solid phase through sorption. However, sorbent
particles do act as depth filters toward particulate matter that is not in true
homogeneous solution in the sample.
The filtering of particulate matter is generally detrimental to the analysis and can lead to
plugging of the extraction sorbent or channeling the flow through the sorbent. It was
summarized that the severity of a plugging problem in SPE depends on
(1) the concentration, type, and size of the particulates in the sample;
(2) the pore size of the sorbent;
(3) the surface area of the sorbent bed.
20.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Methodology
While particulate matter can cause plugging and channeling of the sorbent in SPE as
described above, analysts performing SPE extraction must also be concerned with the
potential for the analyte’s association with particulate and colloidal matter
contamination in the sample.
If the sample is not filtered, particulates can entirely be blocked by the the sorbent, and
subsequently eluted by solvent, leading, when the sample is analyzed, to a result that is
due to the contribution of both dissolved and adhered to particulate analyte. In addition
to concern about the potential for suspended solids in the water sample plugging the SPE
sorbent and analytes of interest adsorbing onto particulates, loss of the analyte may occur
if small particulates pass through the pores of the sorbent bed.
21.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Methodology
If measuring the degree to which the analyte is bound to contaminants in the solution
or, conversely, the degree to which the analyte is unassociated, or in true solution is
important, the sample should be filtered prior to analysis by SPE.
An appropriate level of filtration should be determined for the particular sample matrix
being analyzed and used consistently prior to SPE analysis. The material retained on the
filter may be analyzed separately to determine the level of bound analyte.
22.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Sorbents in SPE – General considerations
(1) a high, reproducible percentage of the analytical solutes must be taken up by the solid
extractant;
(2) the solutes must then be easily and completely eluted from the solid particles.
The sorption process must be reversible.
SPE sorbents should
be porous with large surface areas,
be free of leachable impurities,
exhibit stability toward the sample matrix and the elution solvents,
have good surface contact with the sample solution
23.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Sorbents in SPE – General considerations
(1) nonpolar (and weak polar),
(2) polar,
(3) ion exchange,
(4) chelating agents,
(5) affinity and immunoaffinity,
(6) molecularly imprinted,
(7) restricted access media,
(8) mixed mode,
(9) moisture and particle removal,
(10) monolithic materials,
(11) electrospun nanofibers,
(12) fabrics
24.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
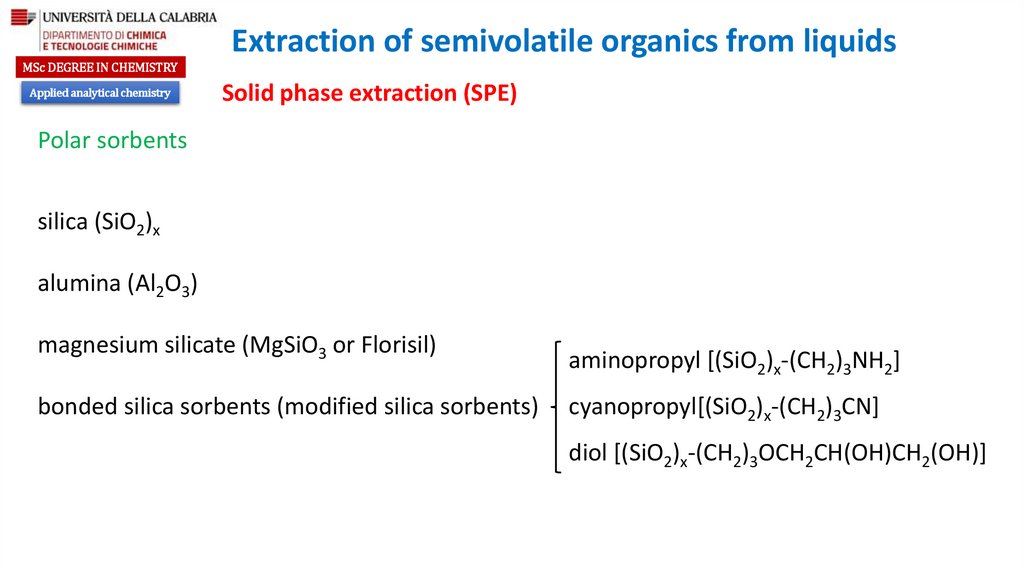
Polar sorbents
silica (SiO2)x
alumina (Al2O3)
magnesium silicate (MgSiO3 or Florisil)
bonded silica sorbents (modified silica sorbents)
aminopropyl [(SiO2)x-(CH2)3NH2]
cyanopropyl[(SiO2)x-(CH2)3CN]
diol [(SiO2)x-(CH2)3OCH2CH(OH)CH2(OH)]
25.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
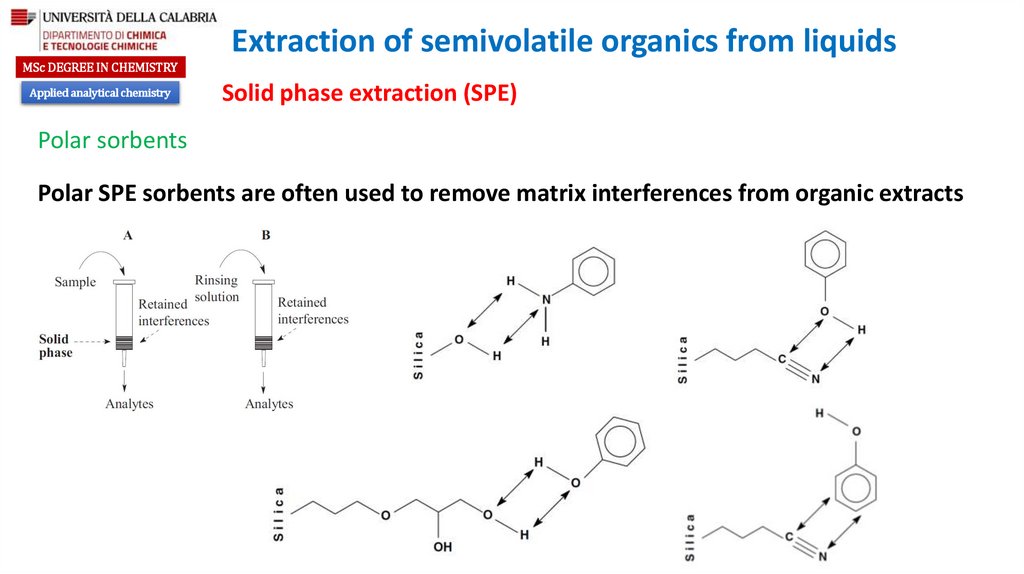
Polar sorbents
Polar SPE sorbents are often used to remove matrix interferences from organic extracts
26.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Polar sorbents
Solid phase extraction (SPE)
27.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Polar sorbents
Silica is one of the best sorbents available for selective separation of compounds with a very similar structure.
The hydrogen bonds and dipole-dipole interactions between silica and polar analytes are strong, especially
when the analyte has functional groups like hydroxyl, carboxyl, or amino. Strong interactions are expected
mainly when the silanol groups are ionized and the analytes are positively charged.
Florisil (MgSiO3) is an extremely polar material, ideal for the isolation of polar compounds from nonpolar
matrices. The larger particle size of the Florisil material enables fast flow of large volume samples and is an
alternative to silica for viscous samples.
Alumina (Al2O3 with free OH groups) is an extremely polar sorbent. The alumina surface tends to be slightly
more stable under high pH conditions than unfunctionalized silica. The smaller particle size ensures high
extraction efficiency, and small sorbent beds are therefore commonly used. This sorbent is usually prepared at
a pH that ensures electrically neutral surface. Neutral alumina is a strongly polar sorbent, which shows good
retention of compounds such as aromatic species and aliphatic amines.
28.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Apolar sorbents
A quite large number of nonpolar SPE materials are commercially available.
- Bonded silica sorbents with nonpolar moieties bonded on surface. One common type
has C18H37 chains bonded (called C18), and some other SPE materials have C8H17 (C8)
chains, phenyl, phenyl-hexyl, etc., bonded groups on the surface.
- Synthetic polymers. A common one being cross-linked polystyrene–divinylbenzene (PSDVB or SDVB) type with no functionalities.
29.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Apolar sorbents
Bonded silica sorbents
Chemically bonded silica sorbents are currently among the most used solid phase for SPE.
Bonded stationary phases are prepared by ‘‘grafting’’ organic nonpolar in this case
(denoted R) to a silica particle via covalent reaction with the silanol groups on its surface.
The importance of this advancement to chromatography in general and particularly to
solid-phase extraction was the ability to produce highly hydrophobic phases that were
very attractive to organic solutes in aqueous solution. Reversed-phase bonded silica
sorbents having alkyl groups covalently bonded to the silica gel backbone interact
primarily with analytes via van der Waals forces. So, contrariwise to polar sorbents, apolar
sorbents are commonly used for extracting semivolatile compounds from aqueous
samples.
30.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Apolar sorbents
Bonded silica sorbents
The bonded phases produced by manufacturers vary according to the nature of the silica
used to prepare the bonded phase and in the reactants and reaction conditions used. The
variations are closely guarded, proprietary manufacturing processes. However, it is
generally known that the most common commercially manufactured bonded-phase
sorbents are based on chemical reaction between silica and organosilanes via the silanol
groups on the silica surface to produce chemically stable Si-O-Si-C covalent linkages to the
silica backbone.
31.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Apolar sorbents
Bonded silica sorbents
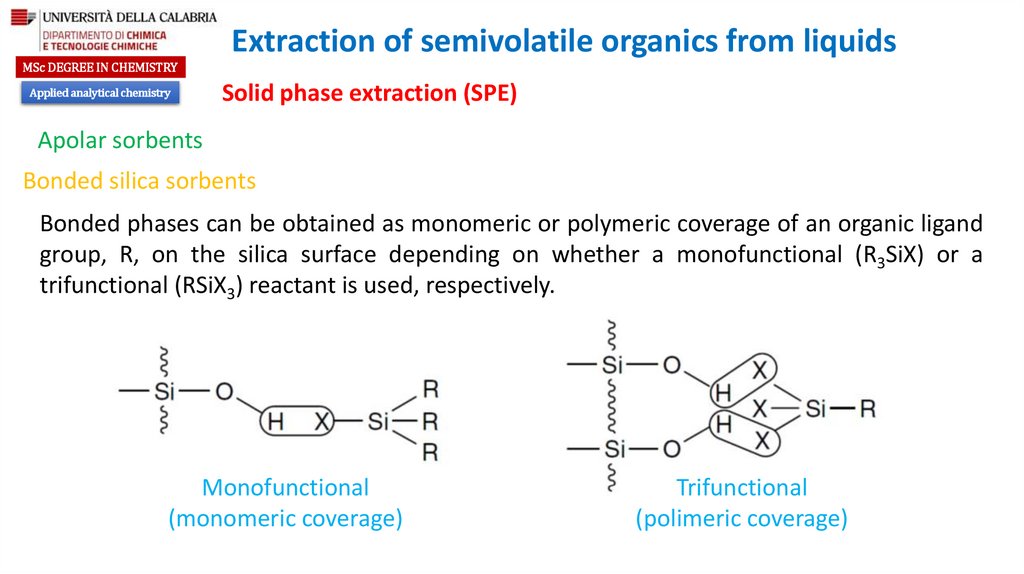
Bonded phases can be obtained as monomeric or polymeric coverage of an organic ligand
group, R, on the silica surface depending on whether a monofunctional (R3SiX) or a
trifunctional (RSiX3) reactant is used, respectively.
Monofunctional
(monomeric coverage)
Trifunctional
(polimeric coverage)
32.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Apolar sorbents
Bonded silica sorbents
33.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Apolar sorbents
Bonded silica sorbents
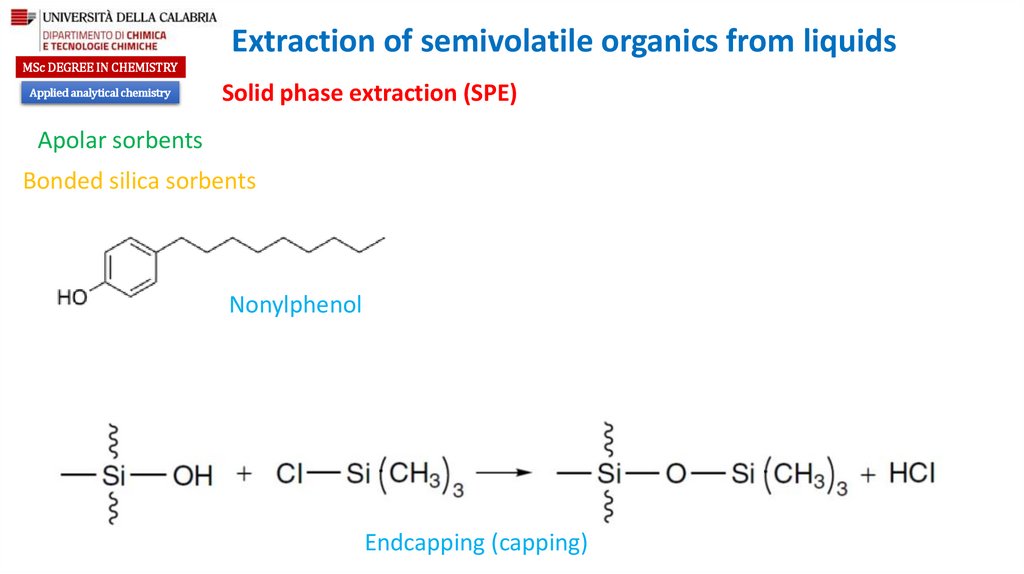
Nonylphenol
Endcapping (capping)
34.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Apolar sorbents
Bonded silica sorbents
For
SPE
derivatized
with
hydrocarbon chains, depending
on the density of the attached
groups and the endcapping
process, the amount of organic
material loaded on the silica base
can be controlled. This amount is
expressed as C% load and can
vary from a few % up to about
18%.
35.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Apolar sorbents
Bonded silica sorbents
The octadecyl type (C18) sorbent has high retentive properties for nonpolar compounds,
and usually has a high carbon load. C18 is generally regarded as the least selective silicabased sorbent, since it retains most organic analytes from aqueous matrices, which is
often a benefit when the compounds of interest vary widely in structure. The potential
for polar interactions between analytes and sorbent is less significant with C18 because of
the predominant effect of the long hydrocarbon chain. However, when the C18 type
sorbent is not endcapped, the remaining silanol groups from the silica backbone may
contribute with polar interactions. The stability of this phase is usually between pH 2 and
9, although progress has been made in extending this range. The narrow pH range is one
of the limitations of silica-based sorbents. At low pH, the alkyl-bonded phases are
susceptible to hydrolysis, and silica dissolution occurs at high pH.
36.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Apolar sorbents
Bonded silica sorbents
C18/OH is a nonendcapped low-load version of the octadecylbonded phases, which
enables the silanol groups on the surface to be more active. This C18 SPE has controlled
silanol activity. The silanol activity enhances retention of basic compounds compared
with endcapped C18. Also, some applications require the retention of compounds with a
wide range of polarities. This may require two types of SPE material and therefore
additional sample preparation processes. Some phases, having more than one type of
group such as C18 and silanol, may be useful for processing a wider range of
compounds.
37.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Apolar sorbents
Bonded silica sorbents
Sorbents with C8 groups are very similar in property to those with C18 but are not as retentive for
nonpolar compounds due to the shorter hydrocarbon chain. C8 can be used as a replacement for
C18 when analytes are too strongly retained on C18 for effective elution. The potential of C8 for
polar interactions with analytes is somewhat higher than for C18 because the shorter
hydrocarbon chain does not mask the silica surface as effectively, but polar interactions are still
not a significant property of C8. The C8 sorbent has been successfully utilized in the simultaneous
extraction of fat and water soluble vitamins from human plasma samples.
Another possible phase contains C2-attached groups. This phase is a fairly polar sorbent because
of the short chain length of the functional groups, which exposes the silica surface. The C2 is
often used as a replacement for C18 and C8 when molecules are retained too strongly by these
phases. This SPE is useful for the extraction of drugs and their metabolites from plasma and urine.
38.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Apolar sorbents
Bonded silica sorbents
Cyclohexyl phase is an average polarity sorbent, which exhibits unique selectivity for
certain solutes such as PAHs. When employed as a nonpolar sorbent, cyclohexyl-silica has
the approximate polarity of a C2 sorbent. The polar subsurface is not an important factor
for cyclohexyl-silica properties. Because of its unique selectivity, CH is often a good
choice when other nonpolar sorbents such as C18, C8, C2, and PH do not provide the
desired selectivity.
Phenyl phase is most commonly employed for nonpolar extractions, with a similar
polarity to a C8 sorbent. Like cyclohexyl, phenyl-silica exhibits a different selectivity from
other nonpolar sorbents. This added selectivity results from the specific interactions with
the aromatic ring. Retention of planar, conjugated organic molecules is enhanced
compared to the aliphatic bonded phases.
39.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Apolar sorbents
Polymeric resins
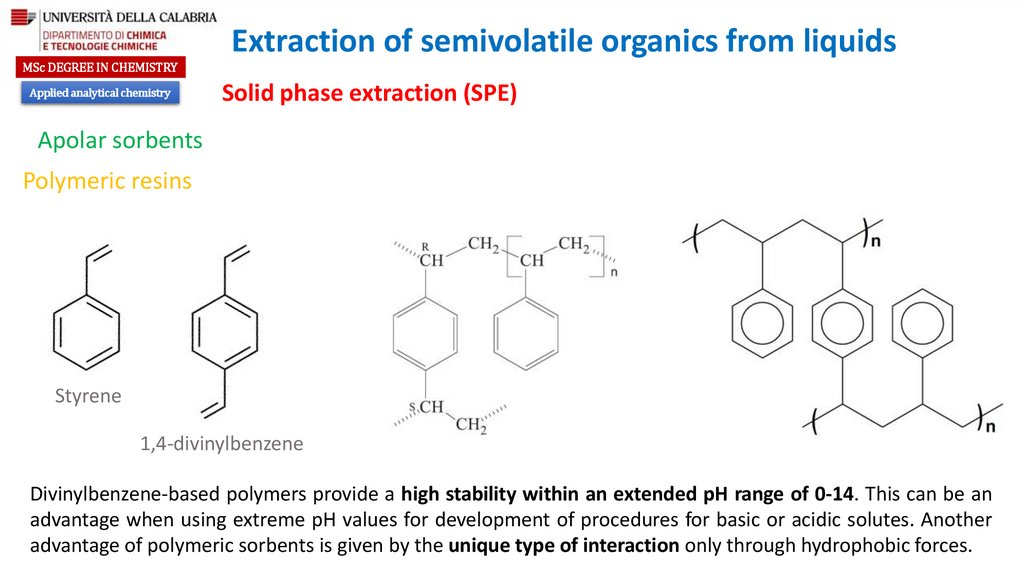
Styrene
1,4-divinylbenzene
Divinylbenzene-based polymers provide a high stability within an extended pH range of 0-14. This can be an
advantage when using extreme pH values for development of procedures for basic or acidic solutes. Another
advantage of polymeric sorbents is given by the unique type of interaction only through hydrophobic forces.
40.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
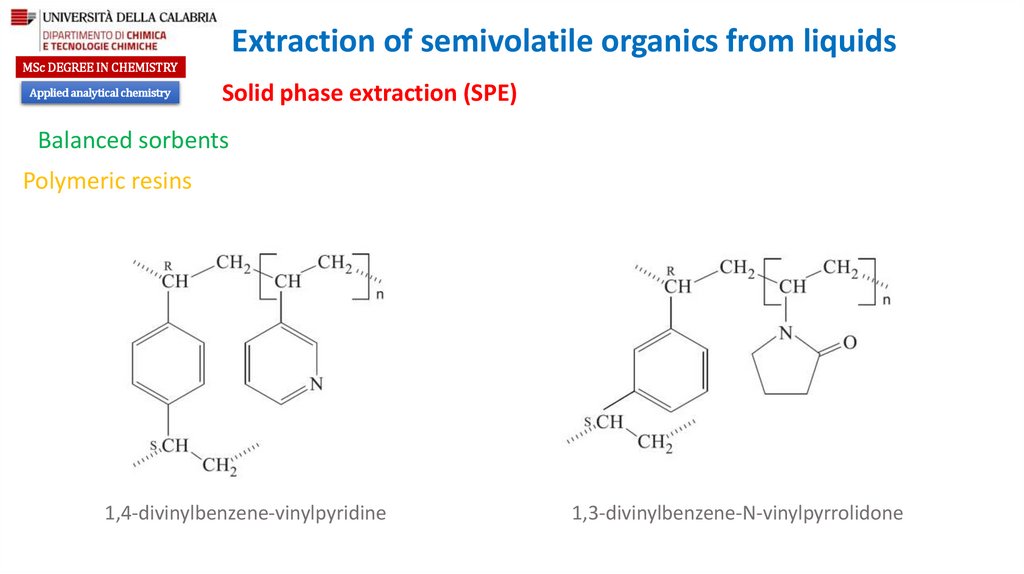
Balanced sorbents
Polymeric resins
1,4-divinylbenzene-vinylpyridine
1,3-divinylbenzene-N-vinylpyrrolidone
41.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
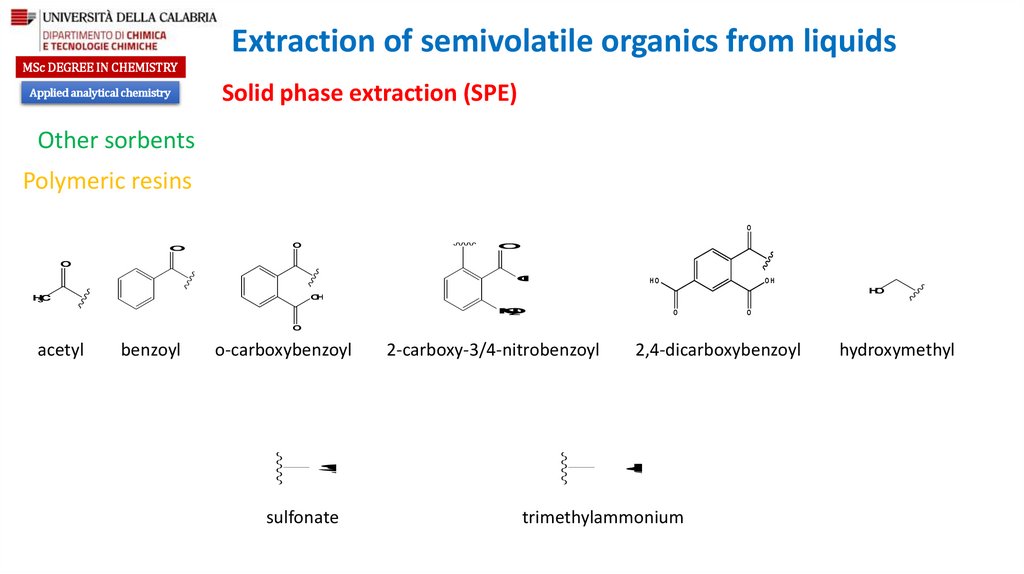
Other sorbents
Polymeric resins
O
O
O
O
O
O
H
HO
OH
H
O
O
H
H
3C
N
O
2
O
O
O
acetyl
benzoyl
o-carboxybenzoyl
2-carboxy-3/4-nitrobenzoyl
S
O
3
sulfonate
2,4-dicarboxybenzoyl
hydroxymethyl
+
N
(
C
H
)
3
3
trimethylammonium
42.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Ion exchange sorbents
Cation exchanger
Cation exchanger
Weak cation exchanger
Anion exchanger
Weak anion exchanger
Anion exchanger
43.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Ion exchange sorbents
The charged functional group on the sorbent associates with the oppositely charged counterion through an
electrostatic interaction.
Cation exchange
Sorbent
Analyte
Anion exchange
Sorbent
Analyte
44.
Extraction of semivolatile organics from liquidsMSc DEGREE IN CHEMISTRY
Applied analytical chemistry
Solid phase extraction (SPE)
Ion exchange sorbents
CBA is often the best
choice
for
cationic
exchange
applications,
especially when dealing
with strong cations (i.e.,
cations with a low pKa).
PRS is most effective for
weaker cations, for which
the neutralization of
analyte can be achieved
in mild pH conditions.
This
nonpolar
character
should
be
taken
into
consideration
when
the
sorbent is used as an ion
exchange for aqueous solvent
systems. This dual nature is
useful
with
compounds
exhibiting both cationic and
nonpolar character.
SAX
offers
minimal
nonpolar
interactions,
because any effect of the
carbon atoms in its
structure is masked by the
quaternary
ammonium
group. Because SAX is such
a strong anion exchanger, it
is a good sorbent for the
retention of weaker anions.


























 Промышленность
Промышленность








