Похожие презентации:
Pneumonia
1. Pneumonia
Y.Gorelik2. What is pneumonia?
An infectious inflammation oflung parenchyma, distal
airways and interstitium.
3. How do we classify pneumonia?
4. Classification
Neither radiological or microbiologicalcriteria are specific for predicting the
cause of pneumonia.
A better approach is to first consider the
clinical circumstances under which
pneumonia acquired
Add the clinical background of the
particular patient
5. How do we classify pneumonia?
Major distinctions (setting of acquisition):Community Acquired Pneumonia (CAP)
Hospital associated pneumonia (HAP)
Health care associated (HCAP)*
Ventilator-associated pneumonia (VAP)
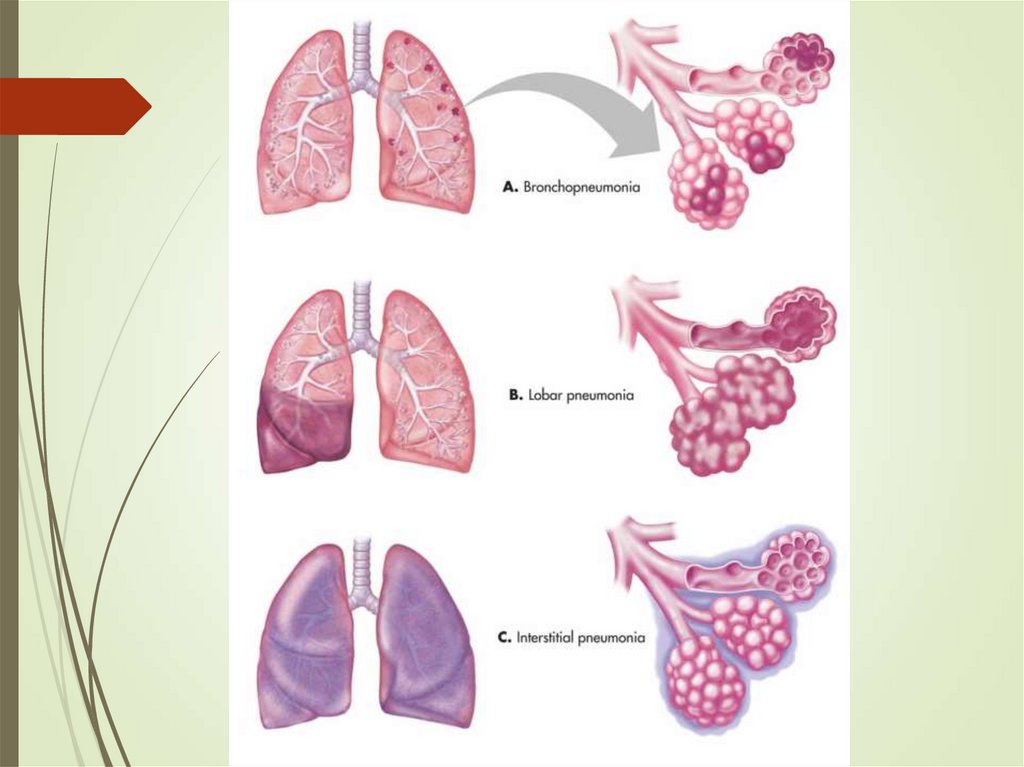
By anatomy or pathophysiology:
Lobar pneumonia
Bronchopneumonia
Interstitial pneumonia
Aspiration pneumonia
Post-obstructive pneumonia
6. Risk Factors for Drug Resistant Pathogens
Hospitalization of >2 d in previous 90 dAntibiotics use in previous 90 d
Immunosupression
Non-ambulatory
Tube feeding
Acid suppression
COPD/bronchiectasis
Hemodialysis
CHF
7.
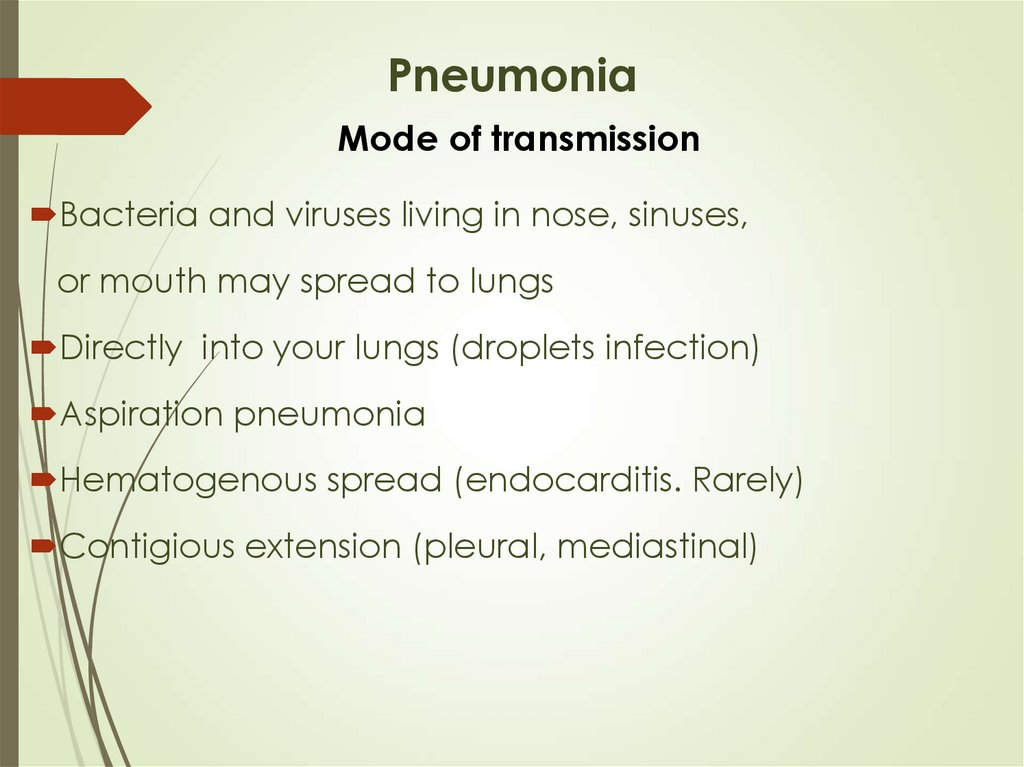
8. Pneumonia Mode of transmission
Bacteria and viruses living in nose, sinuses,or mouth may spread to lungs
Directly into your lungs (droplets infection)
Aspiration pneumonia
Hematogenous spread (endocarditis. Rarely)
Contigious extension (pleural, mediastinal)
9.
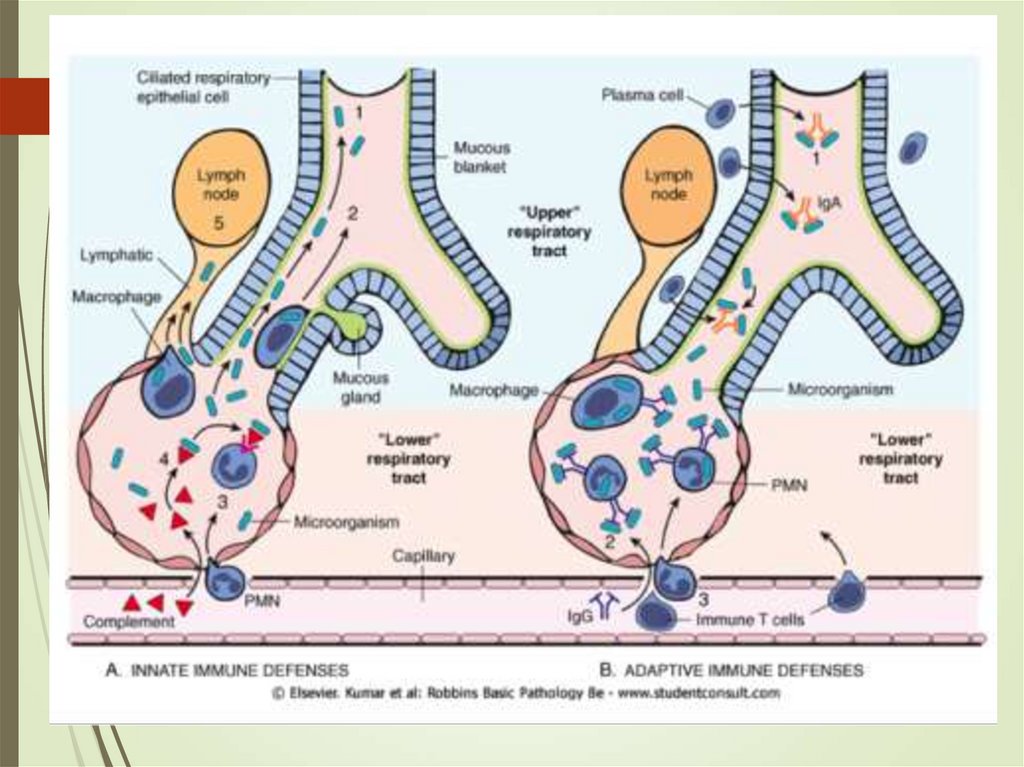
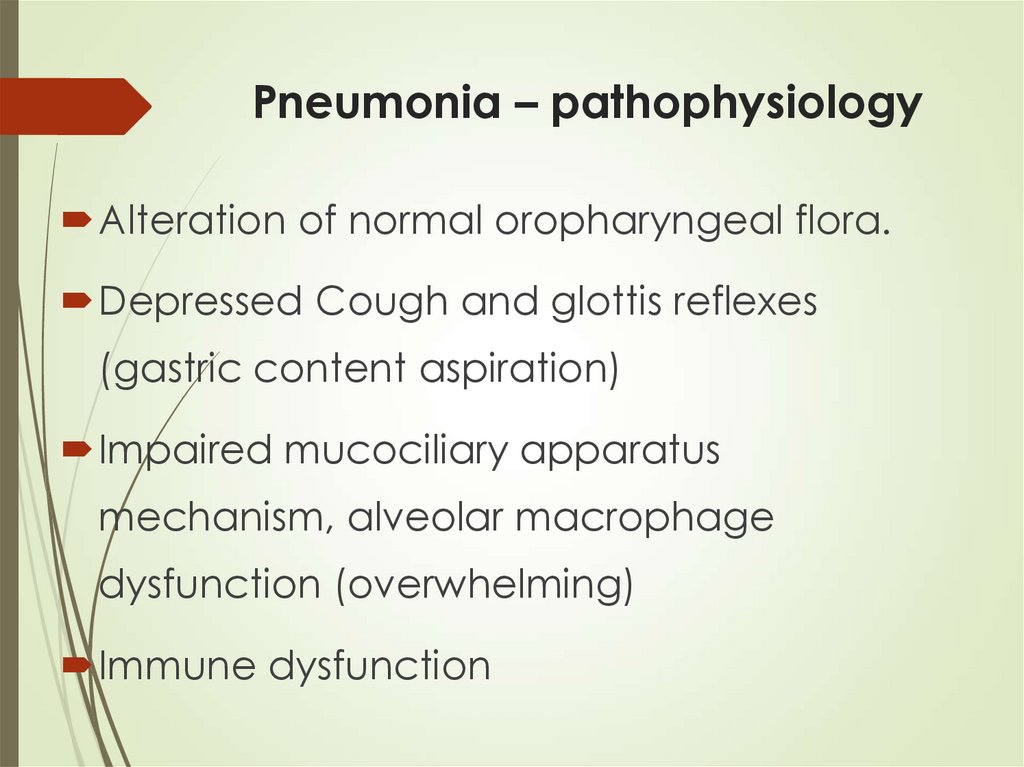
10. Pneumonia – pathophysiology
Alteration of normal oropharyngeal flora.Depressed Cough and glottis reflexes
(gastric content aspiration)
Impaired mucociliary apparatus
mechanism, alveolar macrophage
dysfunction (overwhelming)
Immune dysfunction
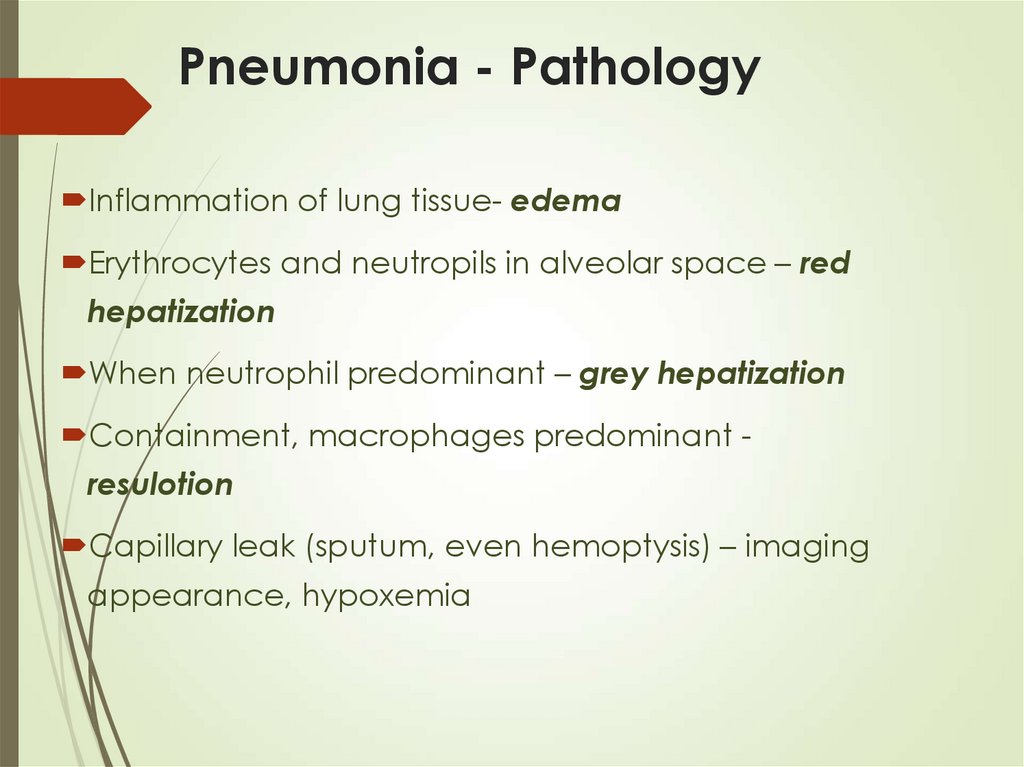
11. Pneumonia - Pathology
Inflammation of lung tissue- edemaErythrocytes and neutropils in alveolar space – red
hepatization
When neutrophil predominant – grey hepatization
Containment, macrophages predominant -
resulotion
Capillary leak (sputum, even hemoptysis) – imaging
appearance, hypoxemia
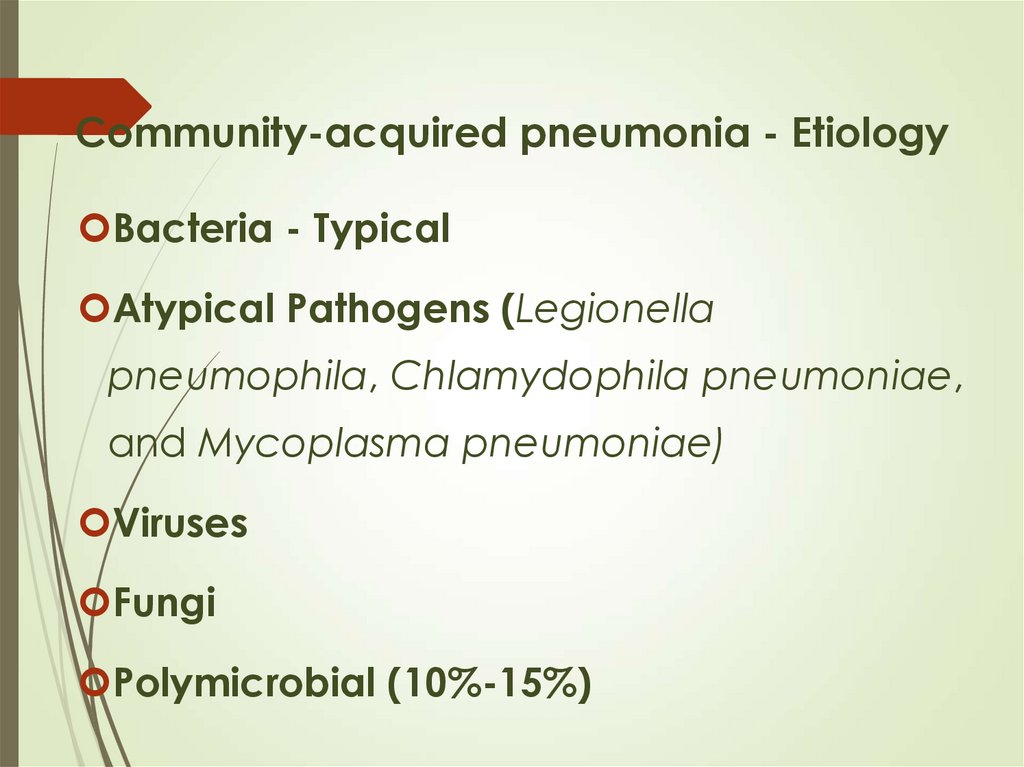
12. Community-acquired pneumonia - Etiology
Bacteria - TypicalAtypical Pathogens (Legionella
pneumophila, Chlamydophila pneumoniae,
and Mycoplasma pneumoniae)
Viruses
Fungi
Polymicrobial (10%-15%)
13. Community-acquired pneumonia
BacteriaS. pneumoniae, Hemophilus influenzae Staphylococcus
aureus, gram negative bacilli
S. pneumoniae was the most common pathogen among
older patients and among those with significant underlying
disease.
Hemophilus infection found in 5%— mostly in patients with
comorbidities.
Staphylococcus aureus - necrotizing pneumonia, abscess
Anaerobs
14. Community-acquired pneumonia
Respiratory viruses (20%-30% inhealthy and patients)
Influenza (A and B)
Human metapneumovirus
Adenovirus
Respiratory syncytial virus
Can precede severe bacterial superimposed
infections
15. Special populations
Alcoholism – S.pneumoniae, anaerobes, K.pneumoniae, Acinetobacter spp., Mycobacterium
tuberculosis (less common locally)
COPD – H. influenzae, P. aeruginosa, Legionella, S.
pneumoniae, M. catarrhalis, C. pneumoniae
Structural diseases - P. aeruginosa, Burkholderia
cepacia, Staphylococcus aureus
Dementia, stroke – Oral anaerobs, enteric bacteria
16. Special populations
Abscess – Staph aureus, anaerobs, fungiShip, hotel, certain establishments – Legionella
Birds (parrots)- chlamidya psittaci
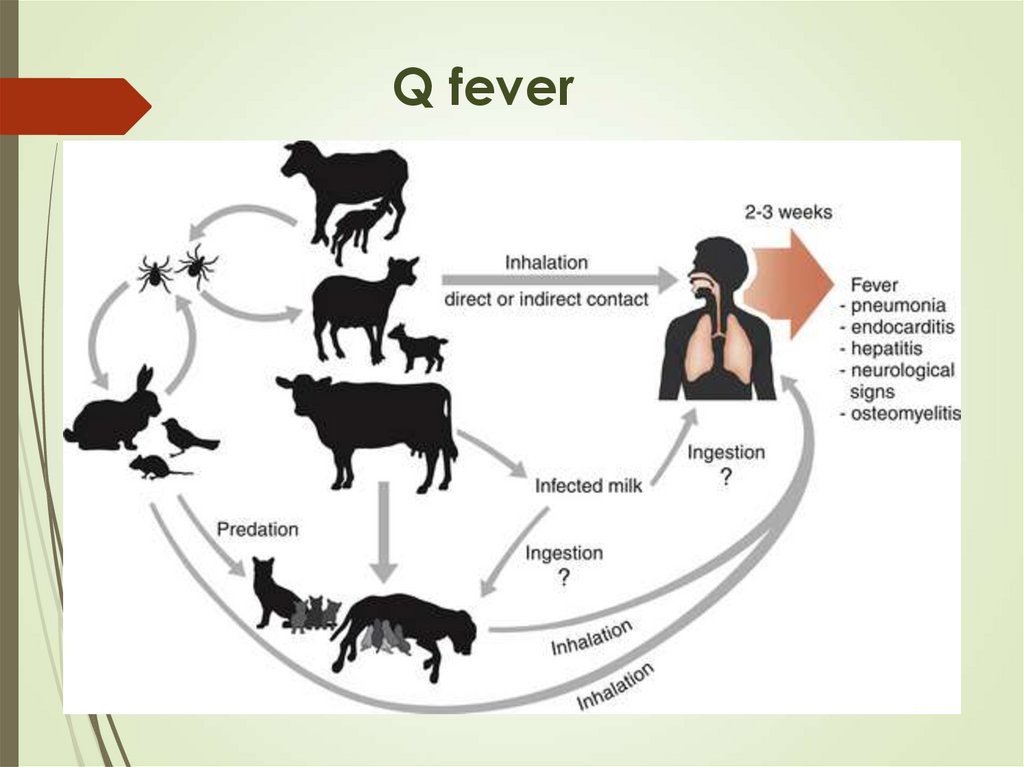
Sheep, goat – Coxiella burnetii
P. aeruginosa – structural lung diseases
Legionella - hematologic malignancy, cancer, severe
renal disease, HIV infection
17. Q fever
18. Clinical manifestations
Abrupt onsetFever chills
Cough (dry, productive, purulent, mucoid)
Dyspnea (uncorrelated with spread)
GI in 20% of patients
Recent associations to MI
19.
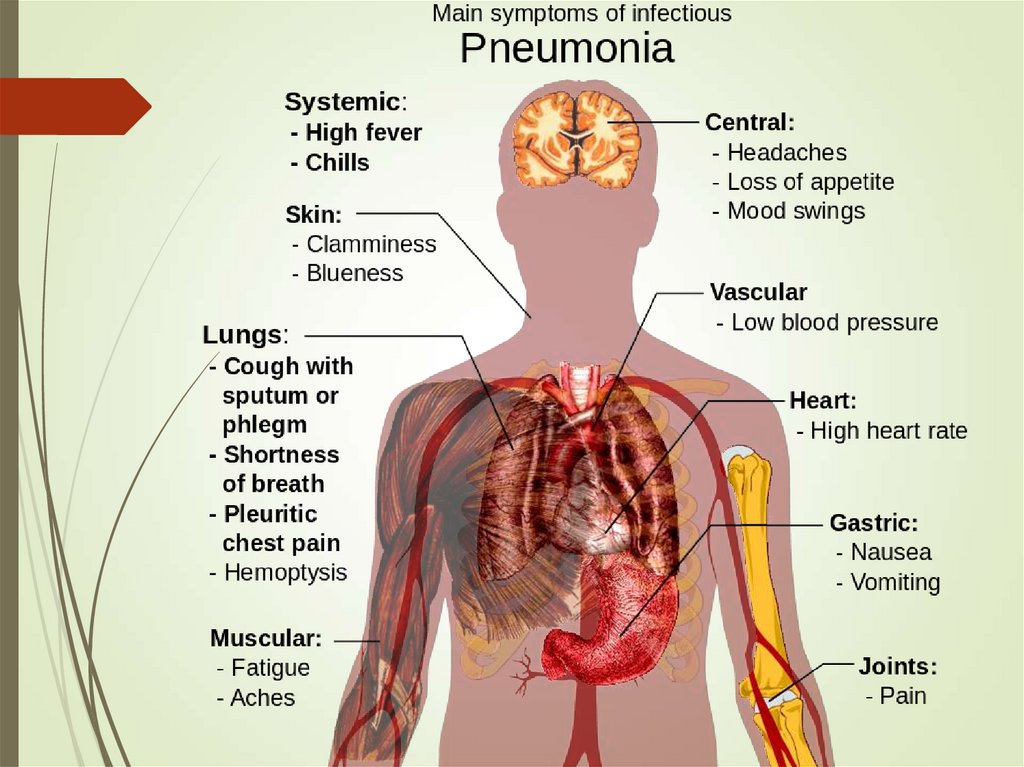
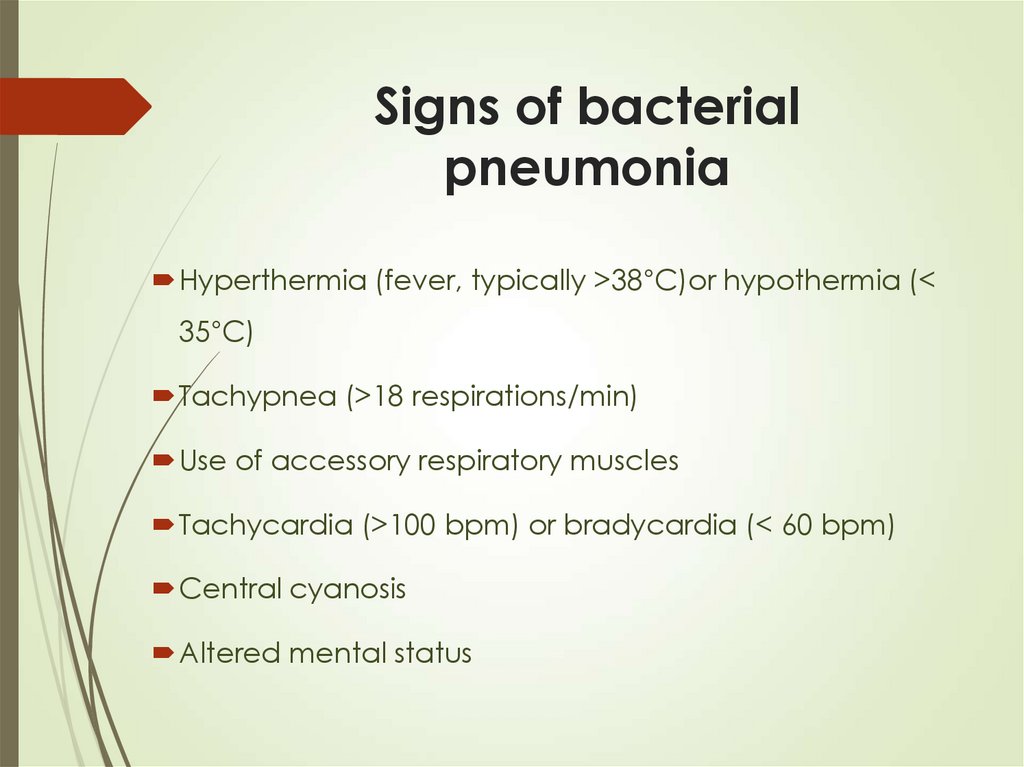
20. Signs of bacterial pneumonia
Hyperthermia (fever, typically >38°C)or hypothermia (<35°C)
Tachypnea (>18 respirations/min)
Use of accessory respiratory muscles
Tachycardia (>100 bpm) or bradycardia (< 60 bpm)
Central cyanosis
Altered mental status
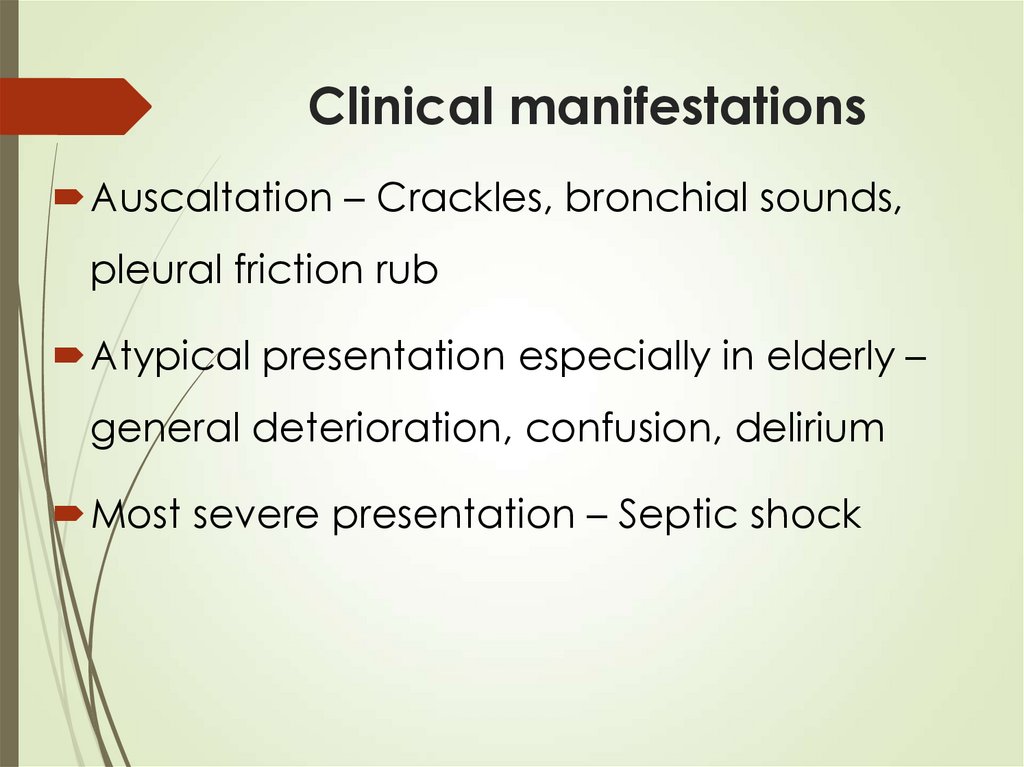
21. Clinical manifestations
Auscaltation – Crackles, bronchial sounds,pleural friction rub
Atypical presentation especially in elderly –
general deterioration, confusion, delirium
Most severe presentation – Septic shock
22. Symptoms
Cough, particularly cough productive of sputum, isthe most consistent presenting symptom of bacterial
pneumonia and may suggest a particular
pathogen, as follows
Streptococcus pneumoniae: Rust-colored sputum
Pseudomonas, Haemophilus, and pneumococcal
species: May produce green sputum
Klebsiella species pneumonia: Red currant-jelly
sputum
Anaerobic infections: Often produce foul-smelling
or bad-tasting sputum
23. Diagnosis of Community-Acquired Pneumonia
Diagnosis of CommunityAcquired PneumoniaSymptoms and signs
Physical exam (sensitivity – 58%,
specificity – 67%)
Laboratory (inflammatory markers)
A new or changed infiltrate on imaging
(sometimes suggestive of etiology)
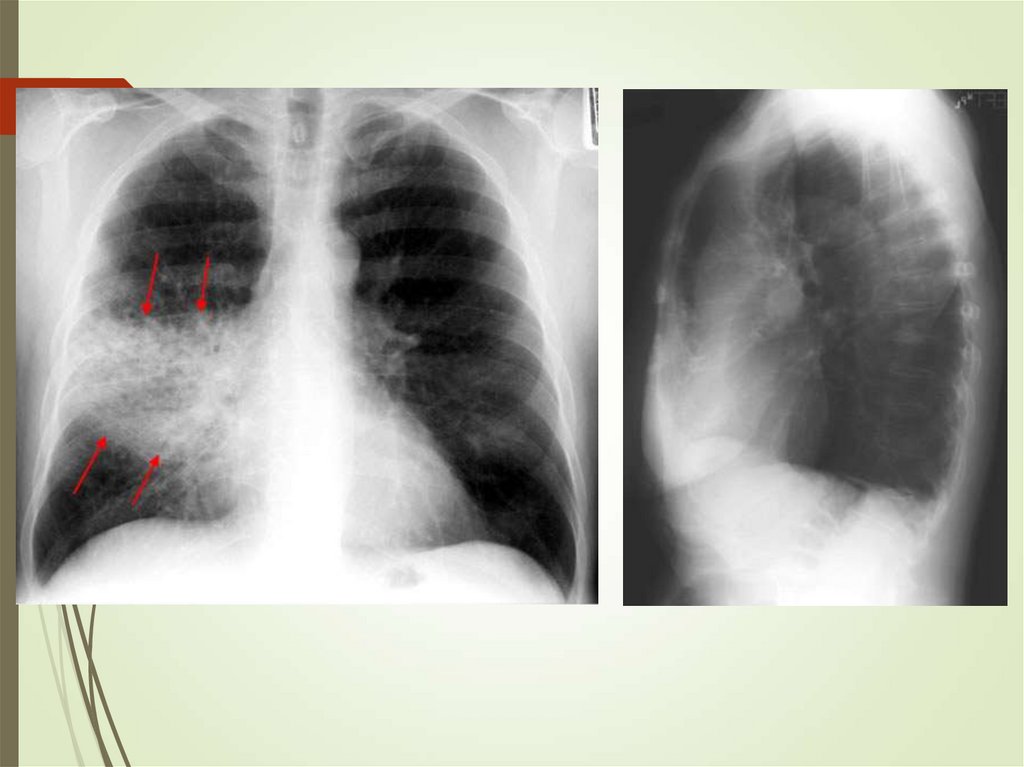
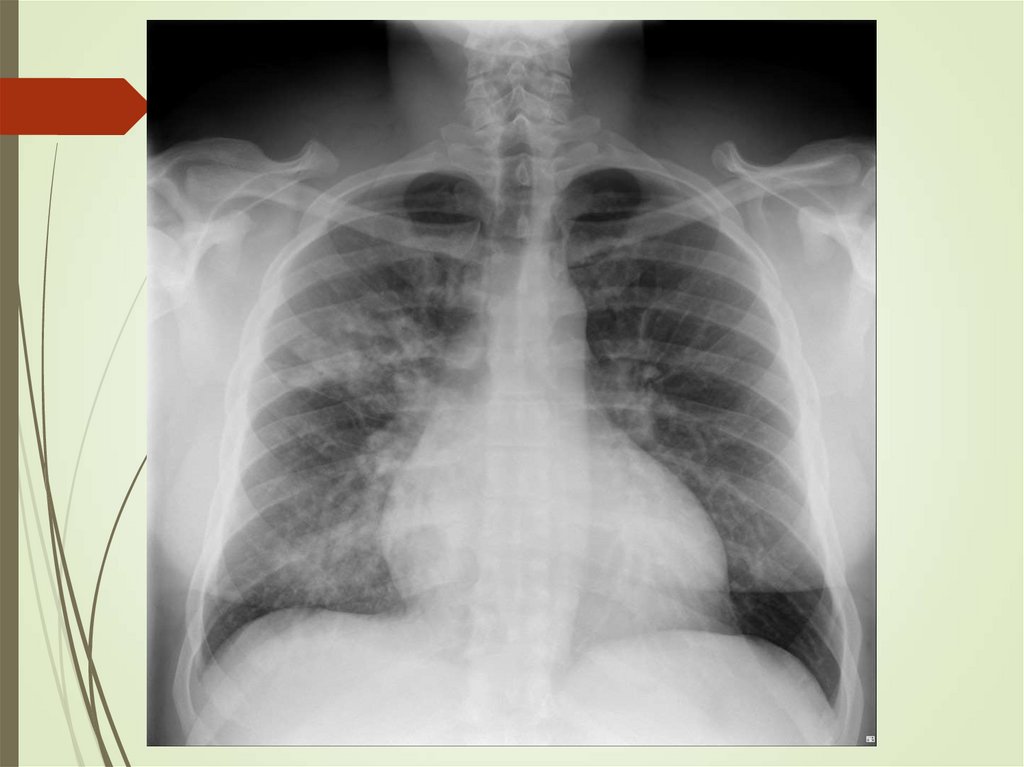
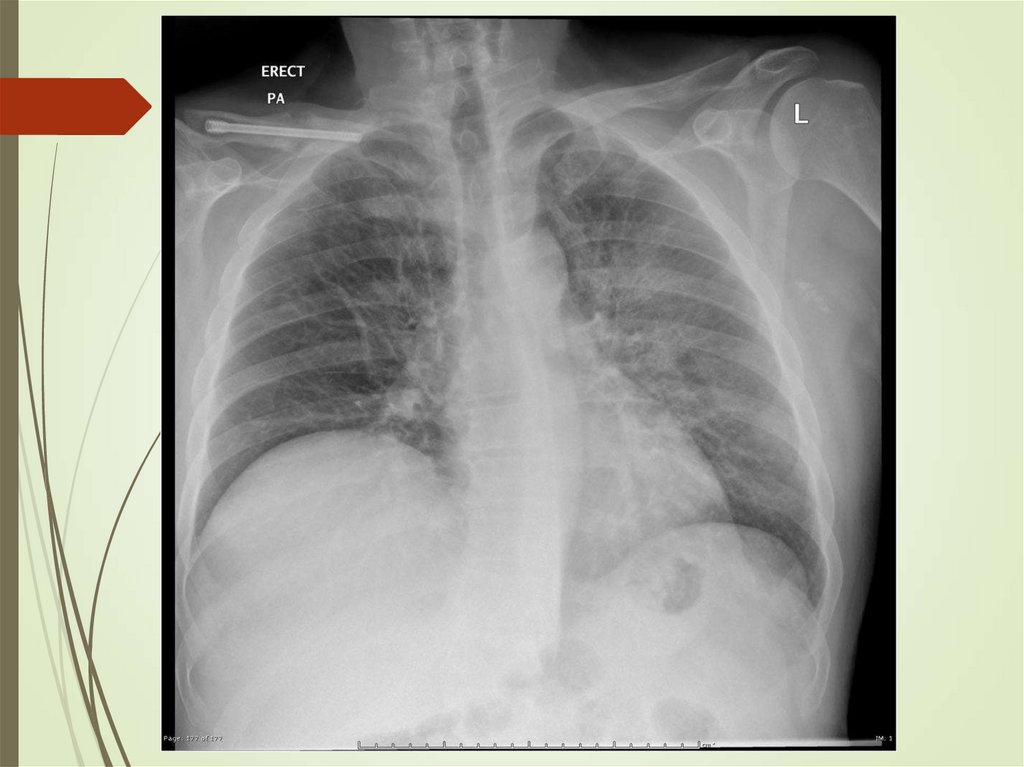
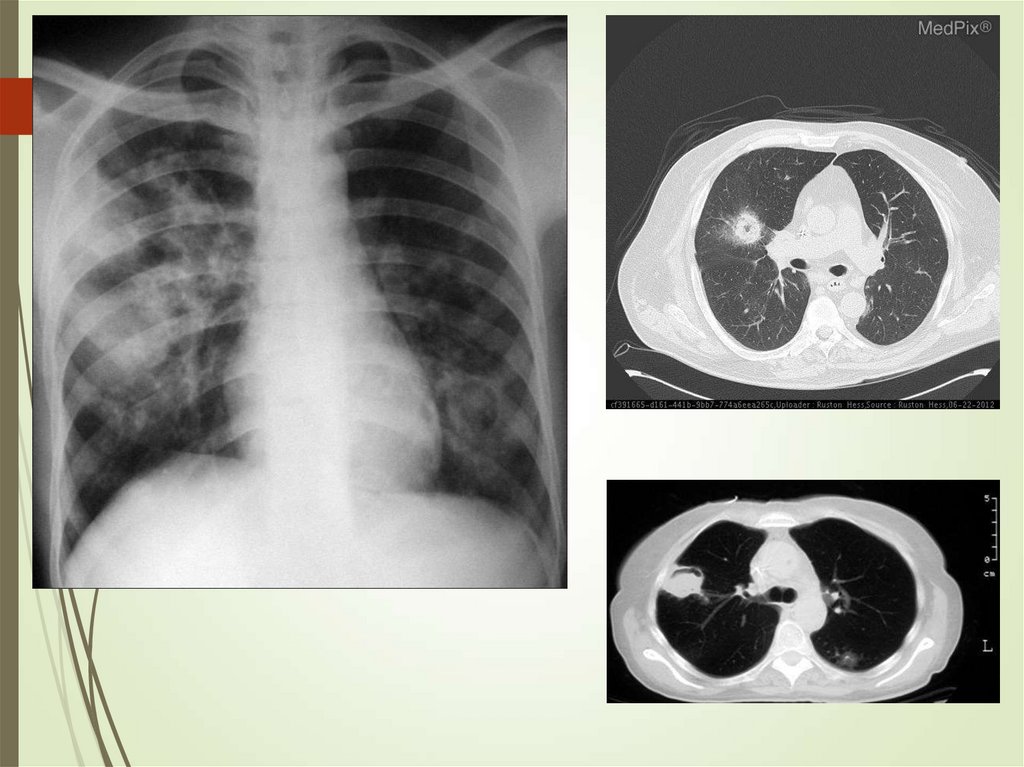
24. Imaging studies
Chest radiography: The criterionstandard for pneumonia diagnosis
Chest computed tomography scanning
Chest ultrasonography
Radiologists may disagree 10% of the
time in diagnosing pneumonia from
chest films.
25.
26.
27.
28.
29. Diagnosing Pneumonia etiology: is it worth the bother?
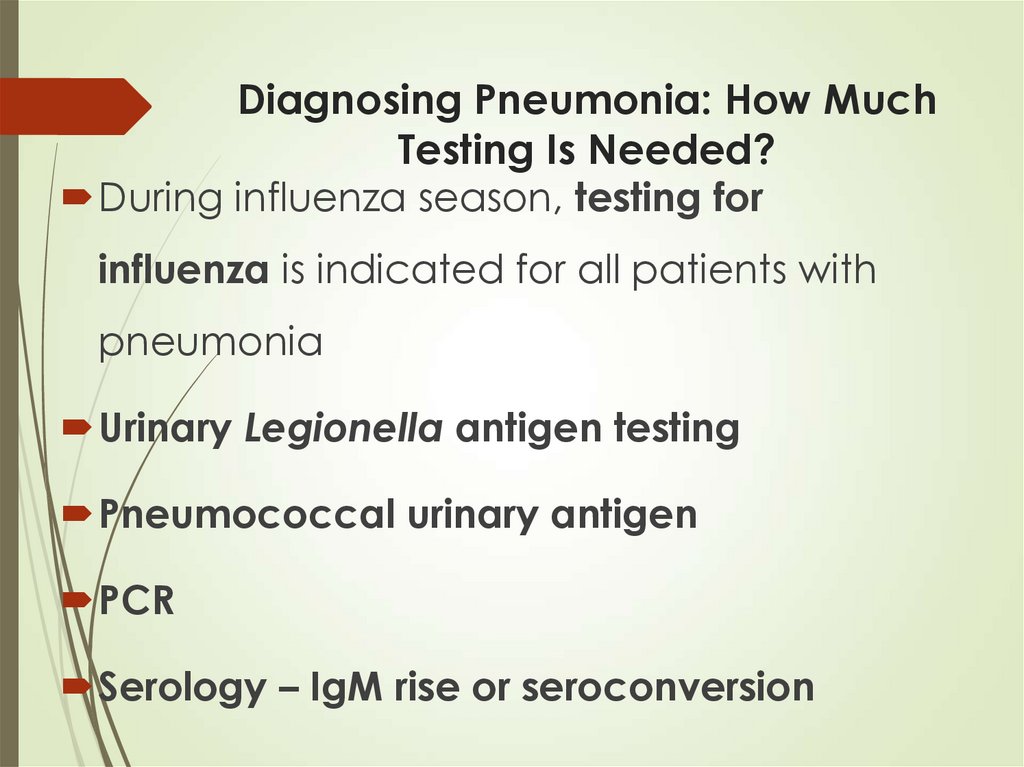
30. Diagnosing Pneumonia: How Much Testing Is Needed?
During influenza season, testing forinfluenza is indicated for all patients with
pneumonia
Urinary Legionella antigen testing
Pneumococcal urinary antigen
PCR
Serology – IgM rise or seroconversion
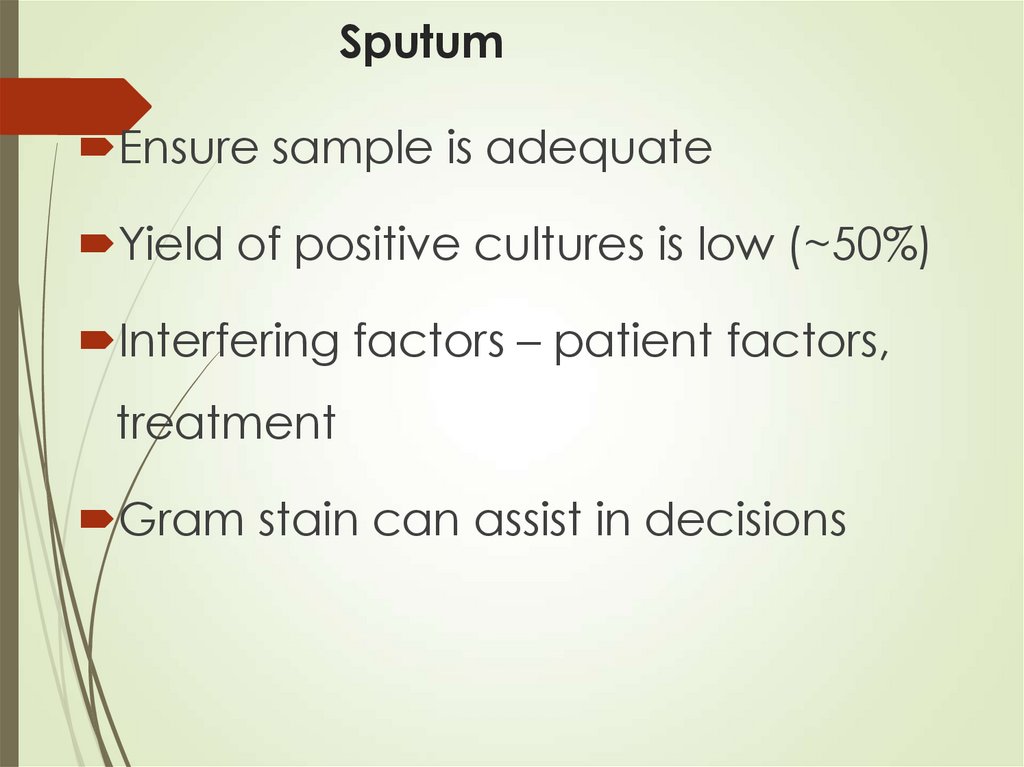
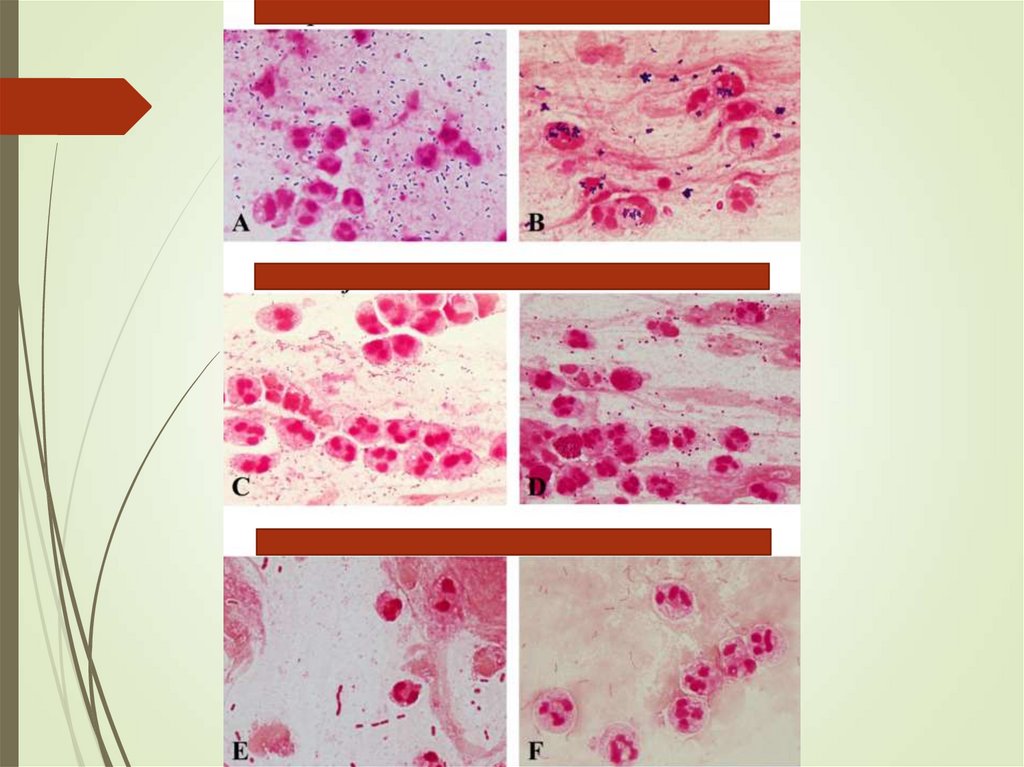
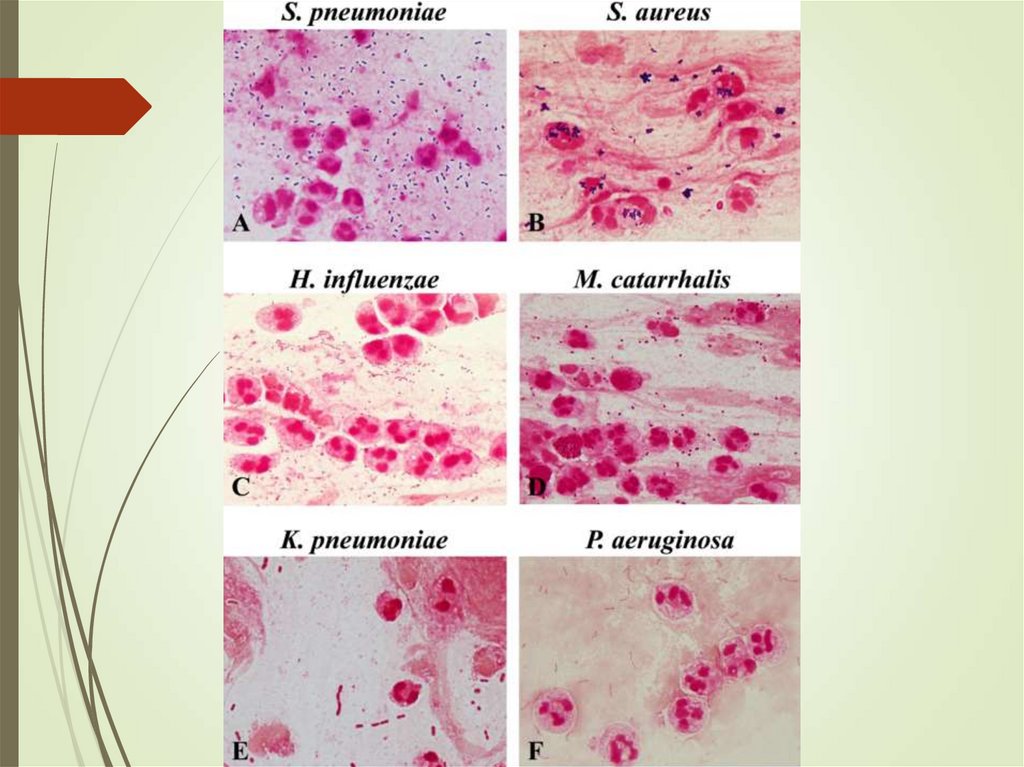
31. Sputum
Ensure sample is adequateYield of positive cultures is low (~50%)
Interfering factors – patient factors,
treatment
Gram stain can assist in decisions
32.
33.
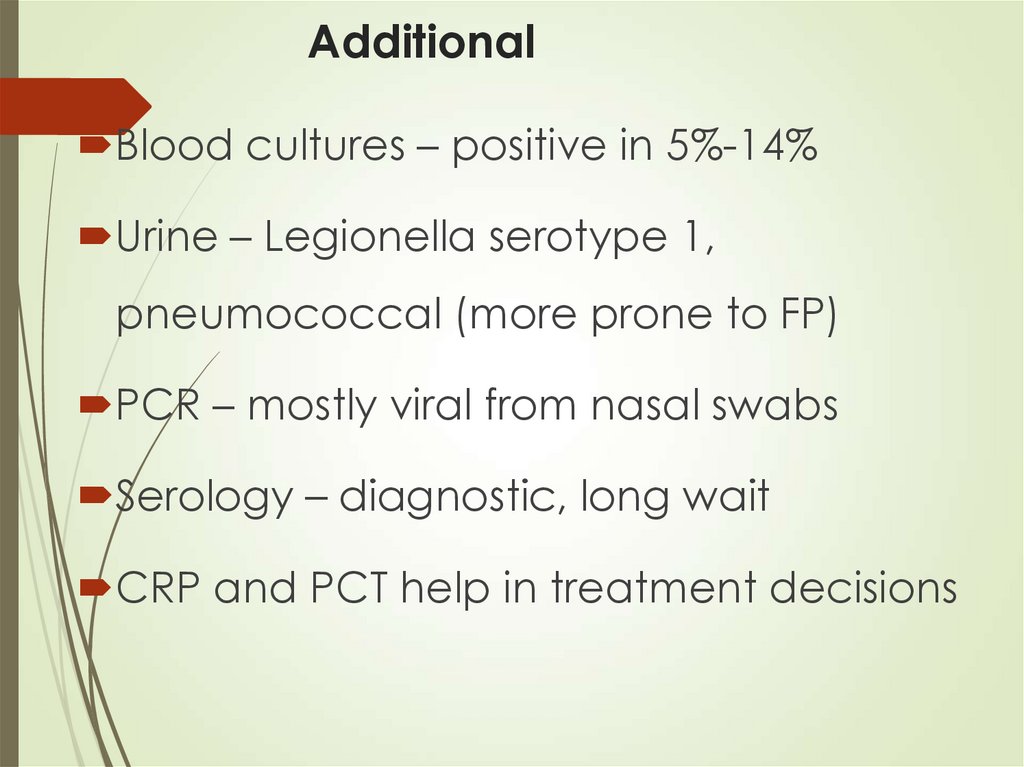
34. Additional
Blood cultures – positive in 5%-14%Urine – Legionella serotype 1,
pneumococcal (more prone to FP)
PCR – mostly viral from nasal swabs
Serology – diagnostic, long wait
CRP and PCT help in treatment decisions
35. Treatment – Main questions
Where?How?
36. British Thoracic Society CAP severity assessment:CURB 65 score
Will help determine where treated (home vshospital), and likely mortality.
Any of: confusion, urea> 7mmol/l (42 mg/dL),
respiratory rate>30/min, blood pressure
systolic <90mmHg diastolic<60mmHg, age>65
years
Low (0-1), moderate (2), high (3+) severity
ICU admission indicated by CURB score of 4-5
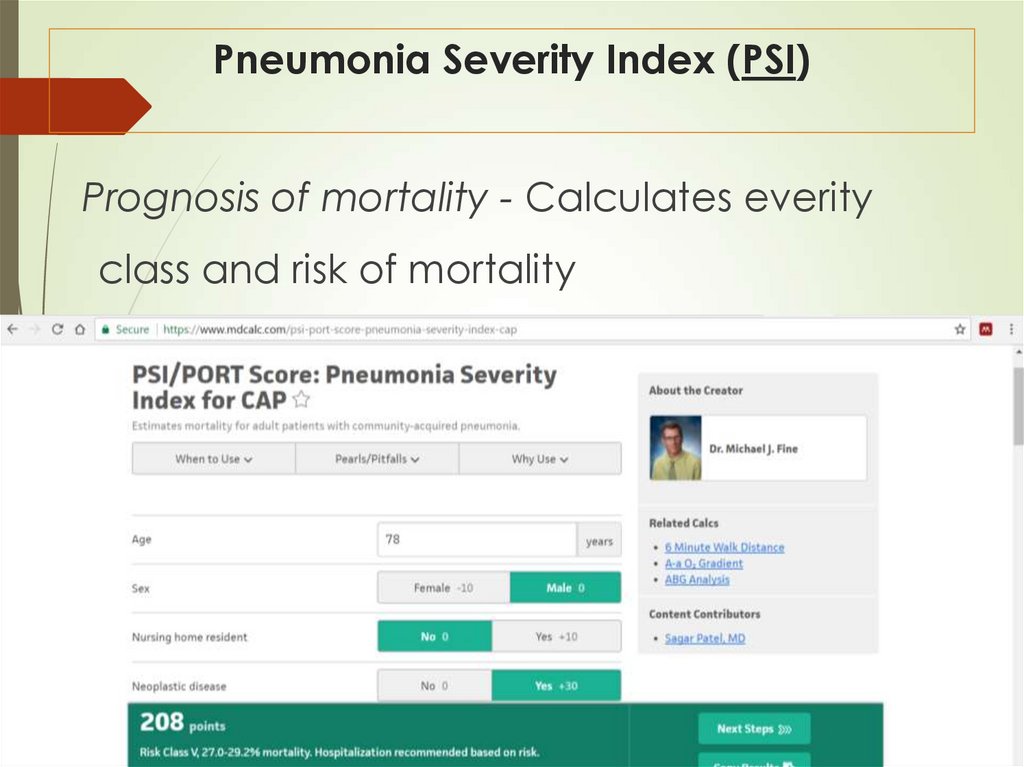
37. Pneumonia Severity Index (PSI)
Prognosis of mortality - Calculates everityclass and risk of mortality
38. Where to Treat Pneumonia: Medical Ward or ICU?
Any patient with 3 of the following 9 criteriabe considered for ICU
1. Confusion
2. BUN ≥20 mg/dL
3. respiratory rate ≥ 30/min
4. multilobar pneumonia
5. hypoxemia with PaO2/FiO2 < 250
6. platelets < 100,000 mm³
7. hypotension (SBP<90 mm Hg)
8. hypothermia < 36º C
9. white blood cell count < 4,000/mm³.
39. Antibiotic Treatment of Community-Acquired Pneumonia
Antibiotic Treatment of CommunityAcquired PneumoniaMain challenge – Resistance
Increasing pneumococcal resistance to β-
lactams
Developing resistance to macrolides,
quinolone and tetracyclines
Gram negatives - usually resistant –
quinolones or carbapenems are often used
(pip/taz)
40. Initial therapy - Outpatient
1. Previously healthy and no antibiotics in past 3months
A macrolide - clarithromycin, azithromycin or
Doxycycline
2. Comorbidities or antibiotics in past 3 months
fluoroquinolone moxifloxacin, levofloxacin or
A β-lactam amoxicillin,amoxicillin/clavulanate;
ceftriaxone, cefuroxime plus a macrolide
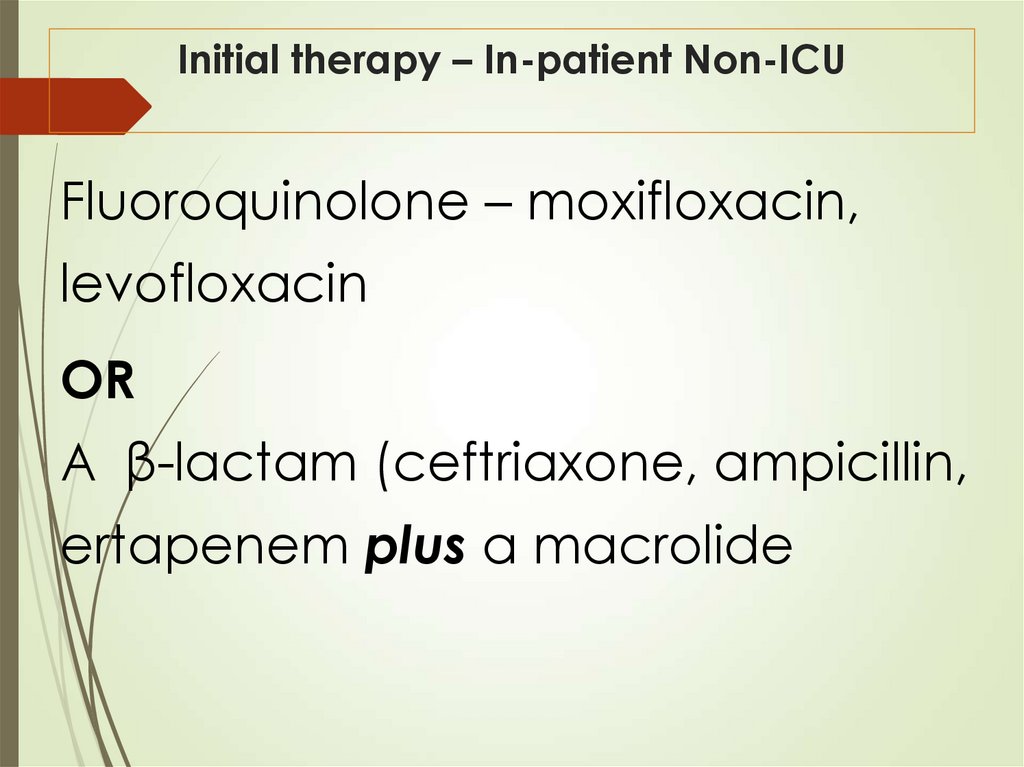
41. Initial therapy – In-patient Non-ICU
Fluoroquinolone – moxifloxacin,levofloxacin
OR
A β-lactam (ceftriaxone, ampicillin,
ertapenem plus a macrolide
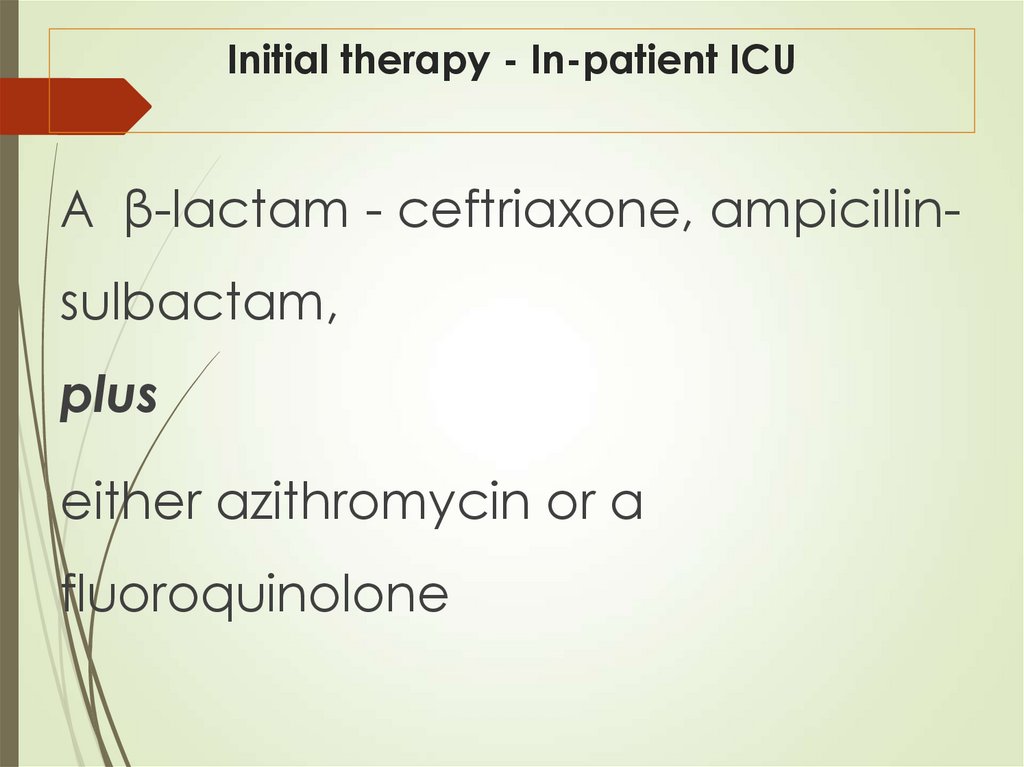
42. Initial therapy - In-patient ICU
A β-lactam - ceftriaxone, ampicillinsulbactam,plus
either azithromycin or a
fluoroquinolone
43. Initial therapy - additional
PseudomonasAn antipseudomonal β-lactam piperacillin/tazobactam,
cefepime, imipenem, meropenem plus either ciprofloxacin or
levofloxacin
The above β-lactams plus an aminoglycoside amikacin or
tobramycin plus azithromycin
The above β-lactamsf plus an aminoglycoside plus a
fluoroquinolone
MRSA - Add linezolid (600 mg IV q12h) or vancomycin
44. Duration of Therapy
Fevere and markers first to improve. Physicalfindings persist longer. X-ray up to 12 weeks
Follow-up x-ray needed!
switched to oral therapy when clinical
improvement, stable and uptaking oral
medicaions
Duration used to be 10-14 days – trials
demonstrated 5 days similar outcomes (even 1
day of ceftriaxone showed substantial results)
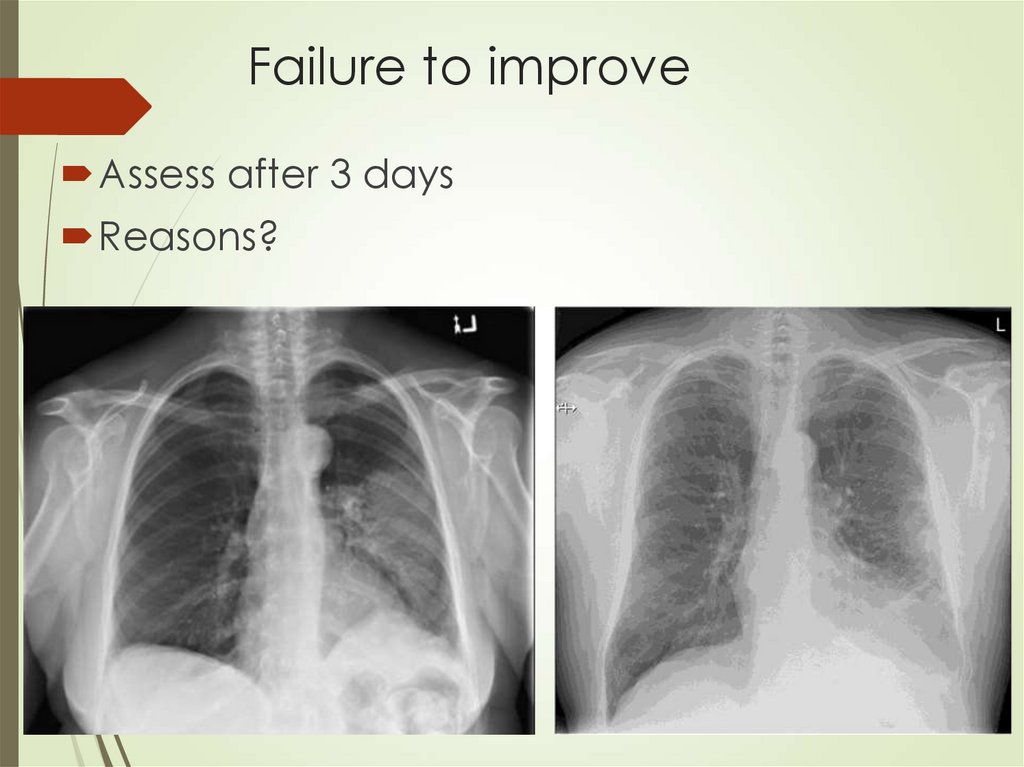
45. Failure to improve
Assess after 3 daysReasons?
46. Complications
Metastatic infection – brain, endocarditisAbscess, empyema. Complete drainage –
pH < 7
LDH > 1000 U/L
Bacteria on stain or culture
Glucose < 40 mg/dL
loculations
47. CAP - Prevention
Influenza VaccinePneumococcal Vaccine – PCV13
for children elderly (>65) and
immunocompromised
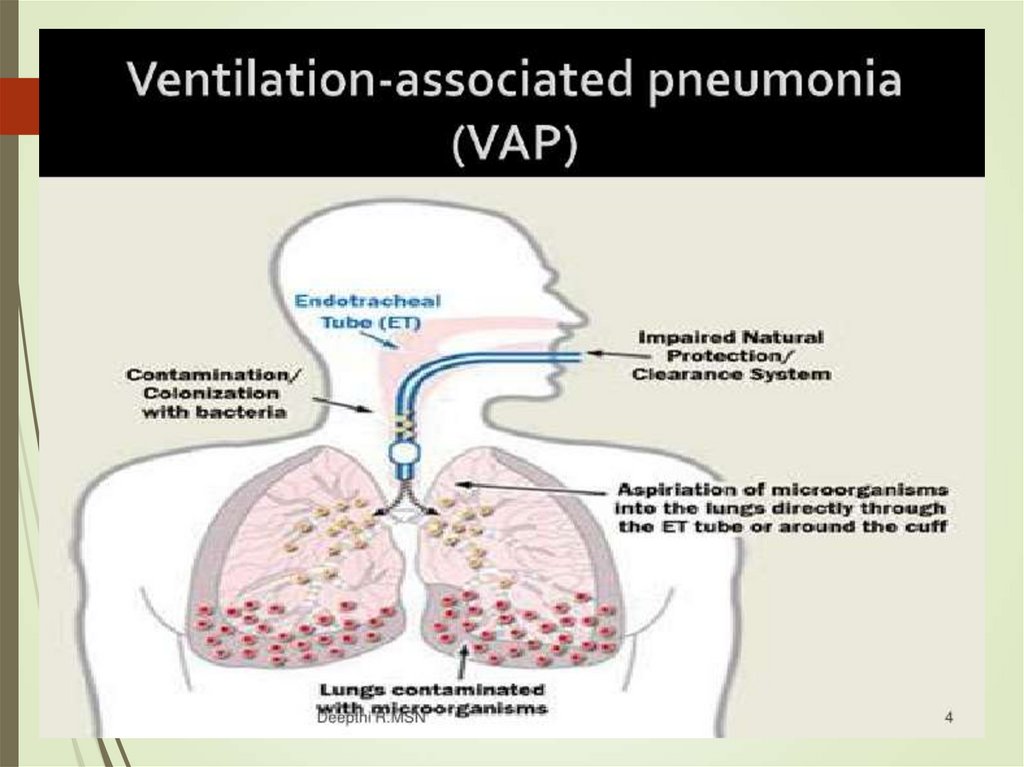
48. VAP
Depends on duration of hospitalization5-7 days – CAP organisms, MSSA,
enterobacterioceae
Later – MDR, MRSA, pseudomonas, ESBL
On any day 10% of patients will have VAP.
70% among ventilated for 30 days
Clinically similar with other meausres of
assessment for intubated patients
49.
50.
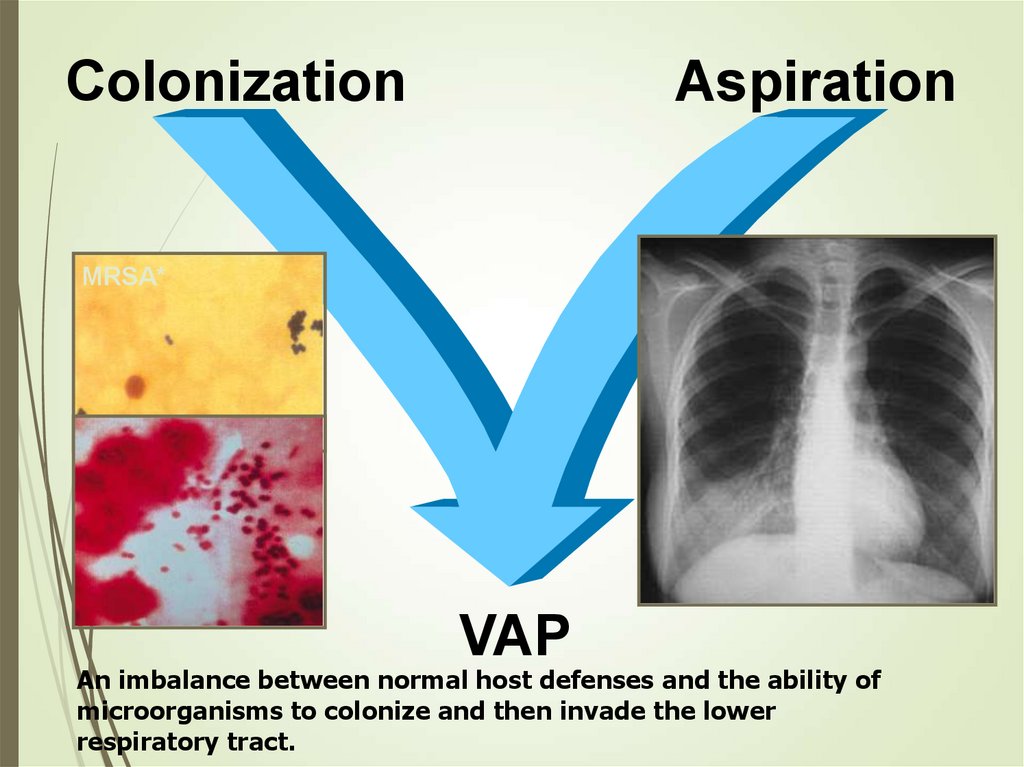
ColonizationAspiration
MRSA*
VAP
An imbalance between normal host defenses and the ability of
microorganisms to colonize and then invade the lower
respiratory tract.
51. VAP - diagnosis
Difficult due to:Bacterial colonization
Misinterpretation and DD to infiltrates
Other possible sources of fever,
respiratory impairments
Debate regarding culture approaches
52. VAP – diagnosis – quantitive culture
Certain threshold of burden defines trueinfection: 10^6 from endotracheal
aspirate and 10^3 from BAL
False negative BAL – location,
interfering therapy
The approach showed lower antibiotic
use and lower mortality
53. VAP – Treatment
Early therapy is essentialAntibiotic selection pressures leads to infections
with highly resistant bacteria
MRSA
Acinetobacter, stenotrephomonas, burkhoderia
P.aurigenosa intrinsically resistant and develops
further resistance during therapy
Almost no atypical (other than legionella)
54. Initial therapy – No MDR risk factors
Ceftriaxone or cefotaxime orMoxifloxacin, ciprofloxacin, or
levofloxacin or
Ampicillin/sulbactam or Ertapenem
55. Initial therapy –MDR risk factors
1. A β-lactam: Ceftazidime or Piperacillin/tazobactamor Imipenem, or meropenem
plus
2. A second agent active against gram-negative:
Gentamicin or tobramycin or amikacin or
Ciprofloxacin or levofloxacin plus
3. An agent active against gram-positive bacterial
pathogens:
Linezolid or Vancomycin
56. VAP – Treatment
Narrow range with culture resultsIf all cultures negative consider stopping
P.auregenosa and MRSA VAP with high
failure rate (50% and 40% respectively)
Most sensitive clinical parameter to
improvement – oxygenation
High crude mortality (50%-70%)
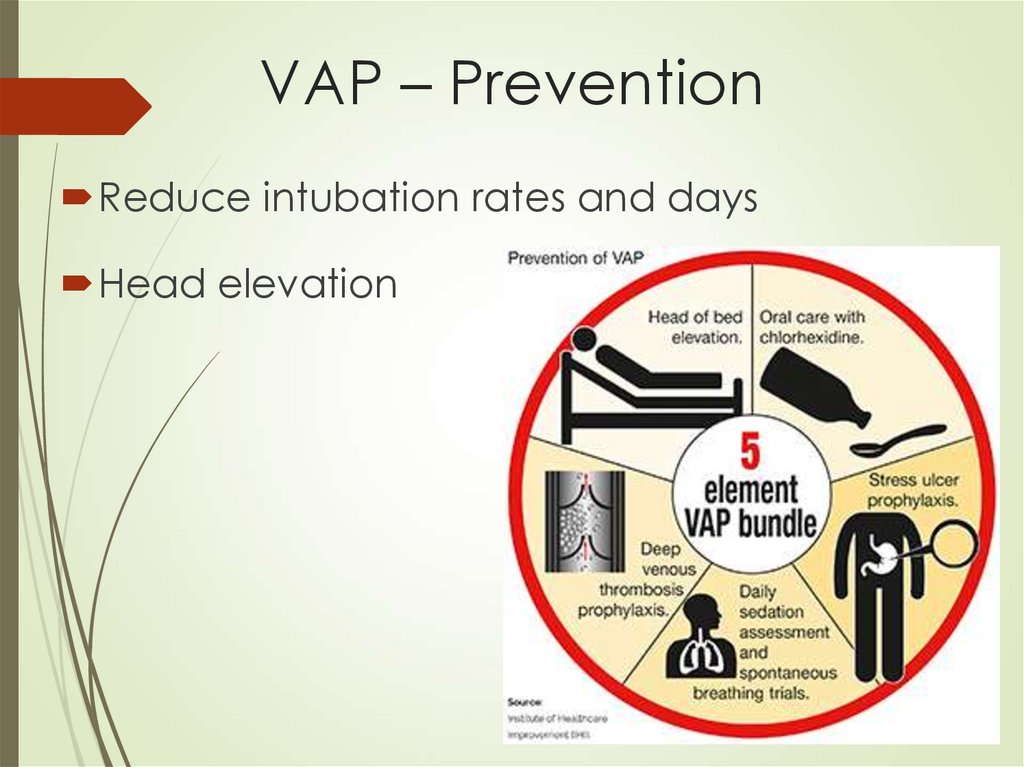
57. VAP – Prevention
Reduce intubation rates and daysHead elevation
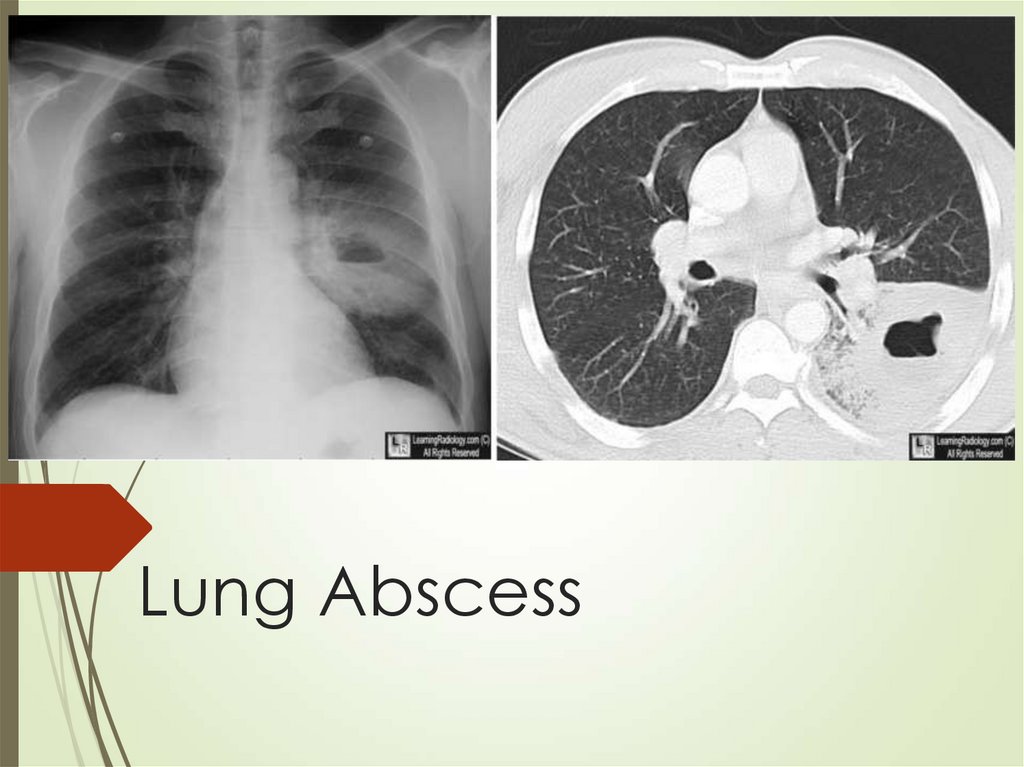
58. Lung Abscess
59. Lung abscess - Etiology
Usually defined as primary (80%) arisingfrom aspiration and secondary from
anatomic or systemic diseases
Major risk factors in population prone for
aspiration
60. Lung abscess
Aspiration causes pneumonitis and thennecrotizing lesions usually infected with
polymicrobial anaerobs
Secondary infections from anatomic
obstructing lesions more commonly with
gram negatives
Clinically more chronic with anemia and
clubbing in addition to other pneumonia
features
61. Lung abscess
Diagnosis usually made with CT thatestablished location and type
Procedures like bronchoscopy or
needle aspiration – spillage risk
62. Lung abscess - Treatment
Primary – gram positive and anaerobs coverage –clindamycin or amoxicillin/clavulanate
Secondary – by organism
Around 7 days for response
Long duration of therapy
10%-20% do not respond especially in large lesions
2% mortality in primary and around 75% in
secondary
63. Pulmonary infections in immunocompromised patients
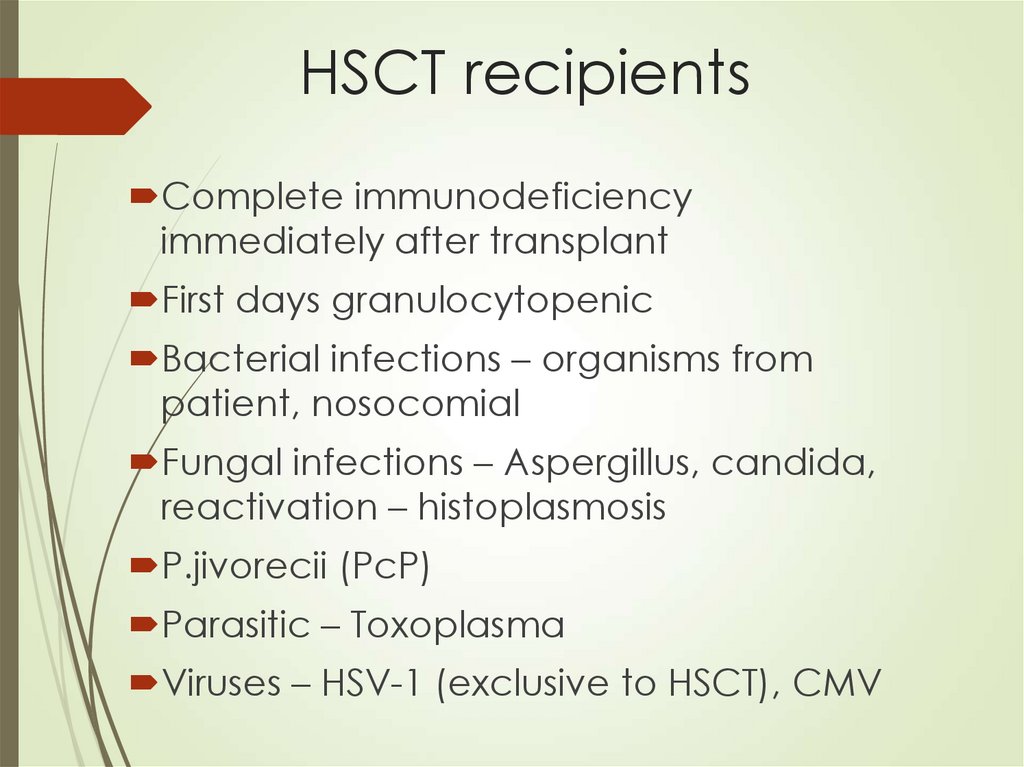
64. HSCT recipients
Complete immunodeficiencyimmediately after transplant
First days granulocytopenic
Bacterial infections – organisms from
patient, nosocomial
Fungal infections – Aspergillus, candida,
reactivation – histoplasmosis
P.jivorecii (PcP)
Parasitic – Toxoplasma
Viruses – HSV-1 (exclusive to HSCT), CMV
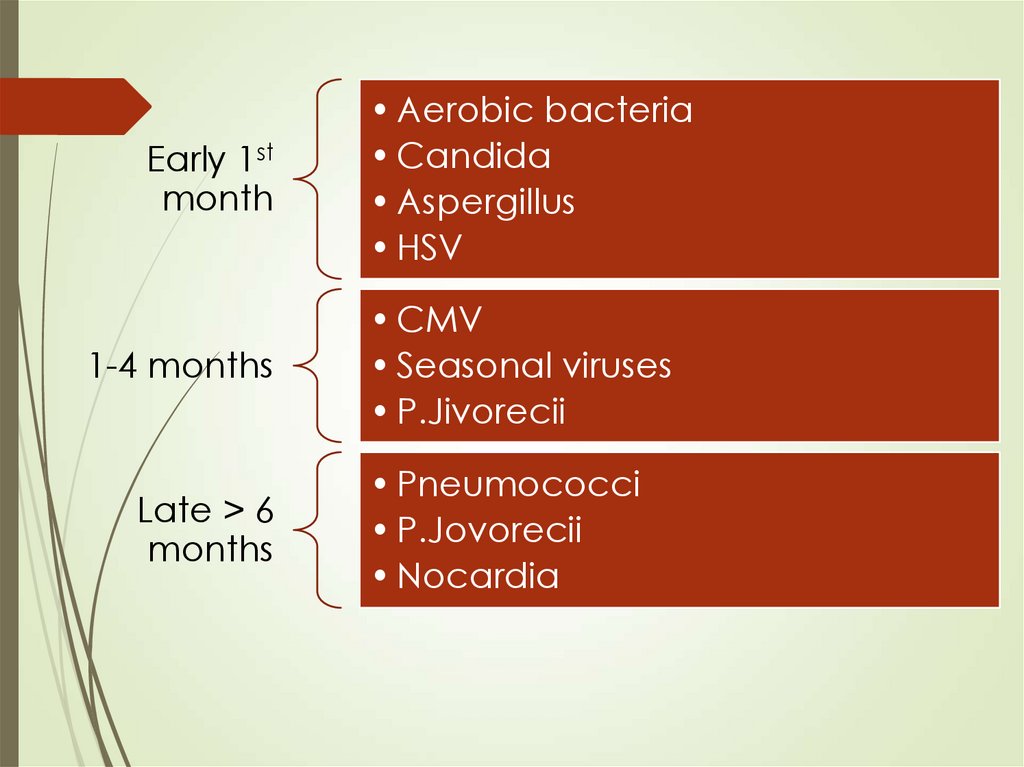
65.
Early 1stmonth
1-4 months
Late > 6
months
• Aerobic bacteria
• Candida
• Aspergillus
• HSV
• CMV
• Seasonal viruses
• P.Jivorecii
• Pneumococci
• P.Jovorecii
• Nocardia
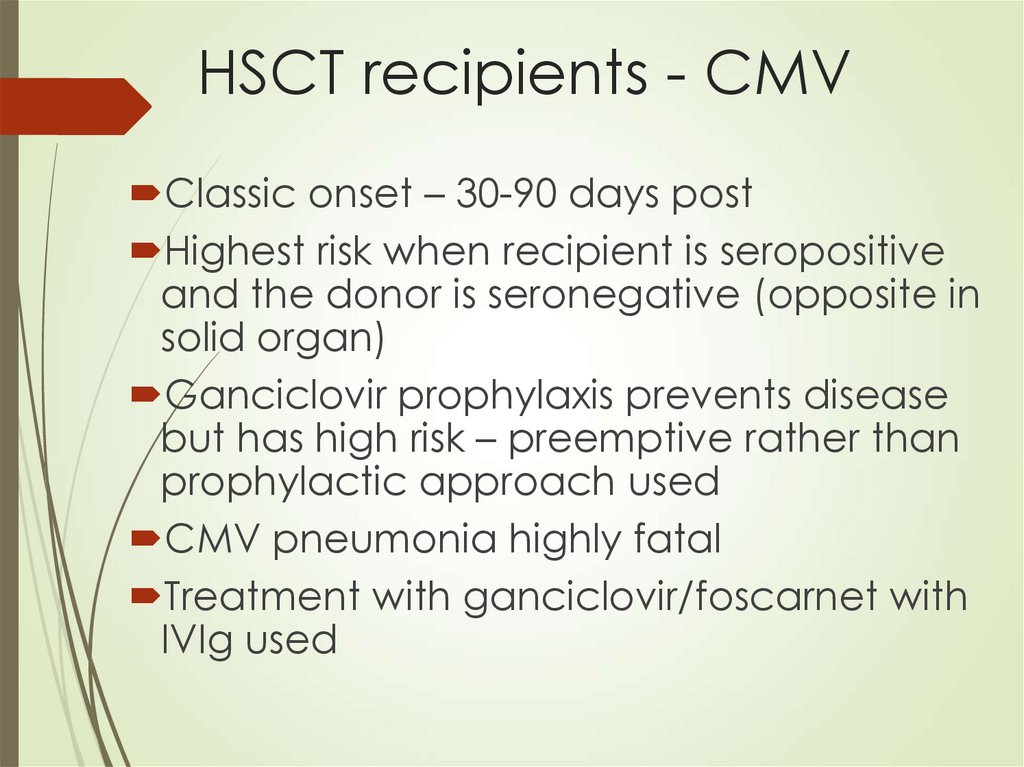
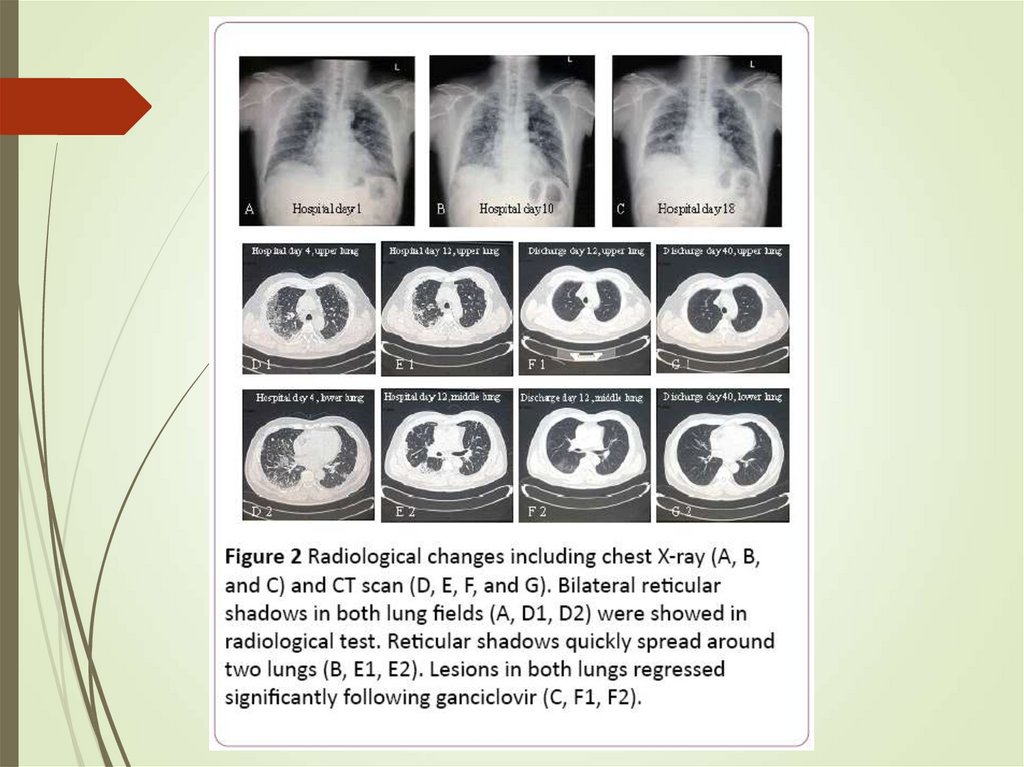
66. HSCT recipients - CMV
Classic onset – 30-90 days postHighest risk when recipient is seropositive
and the donor is seronegative (opposite in
solid organ)
Ganciclovir prophylaxis prevents disease
but has high risk – preemptive rather than
prophylactic approach used
CMV pneumonia highly fatal
Treatment with ganciclovir/foscarnet with
IVIg used
67.
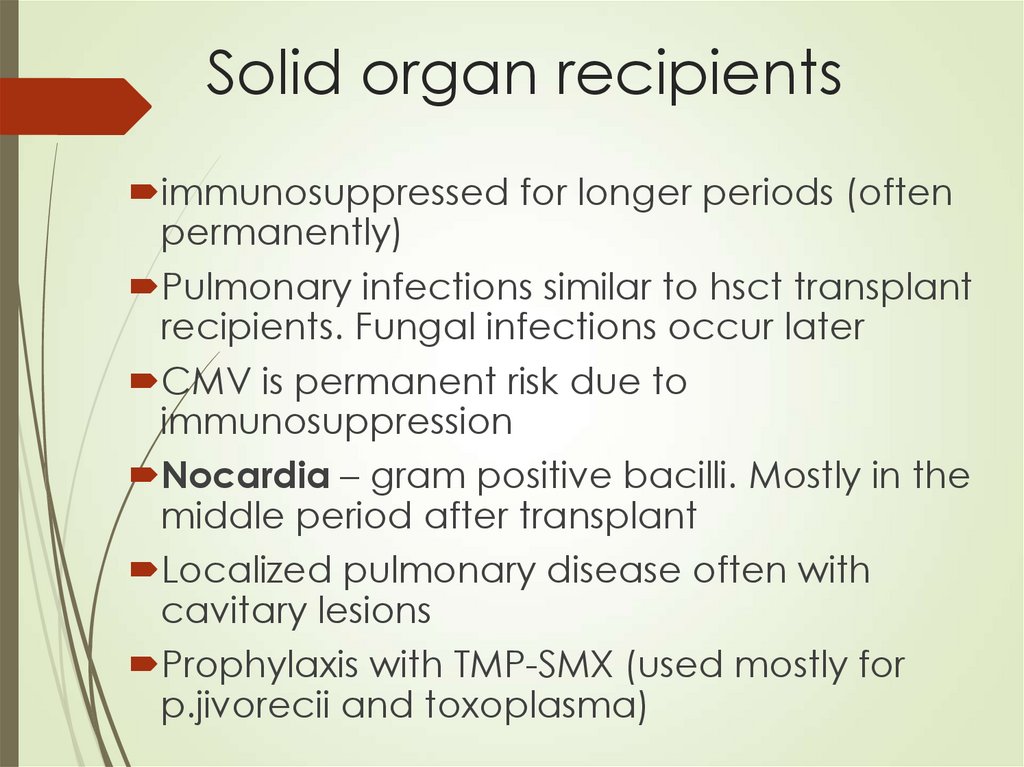
68. Solid organ recipients
immunosuppressed for longer periods (oftenpermanently)
Pulmonary infections similar to hsct transplant
recipients. Fungal infections occur later
CMV is permanent risk due to
immunosuppression
Nocardia – gram positive bacilli. Mostly in the
middle period after transplant
Localized pulmonary disease often with
cavitary lesions
Prophylaxis with TMP-SMX (used mostly for
p.jivorecii and toxoplasma)
69.
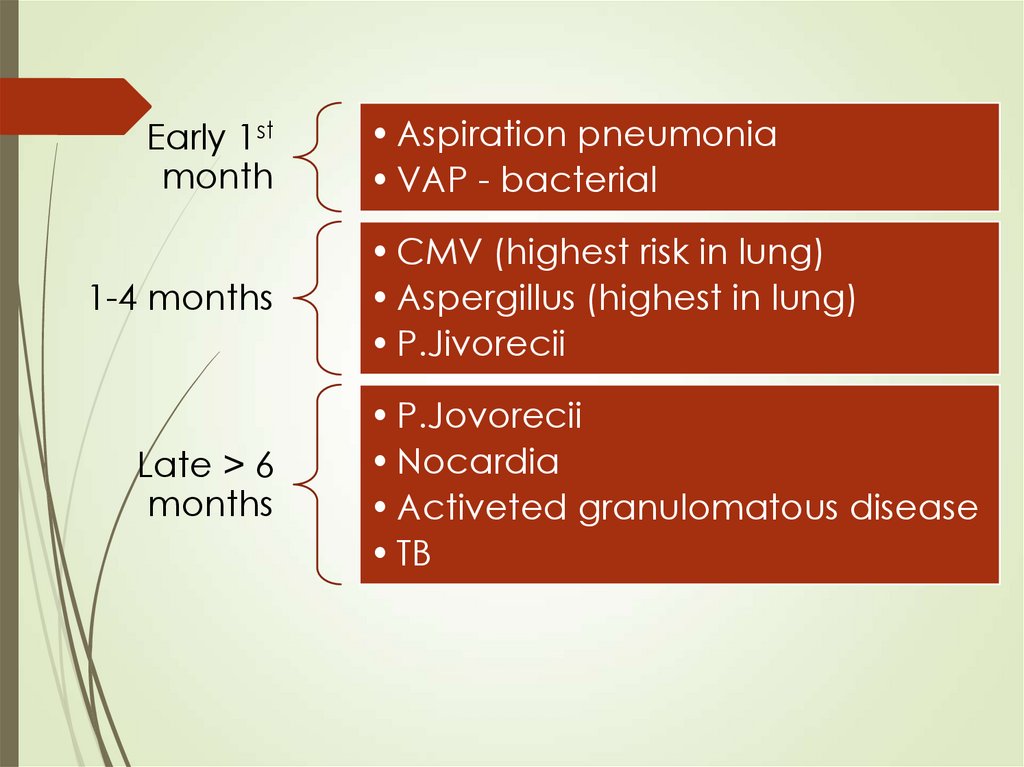
Early 1stmonth
1-4 months
Late > 6
months
• Aspiration pneumonia
• VAP - bacterial
• CMV (highest risk in lung)
• Aspergillus (highest in lung)
• P.Jivorecii
• P.Jovorecii
• Nocardia
• Activeted granulomatous disease
• TB
70.
71. Human immunodeficiency virus associated pulmonary infections
72. HIV – respiratory disease
Recurrent pneumonia, tuberculosis andp.jivorecii pneumonia among most
common AIDS defining illnesses
Pneumococcal - most common bacterial
pneumonia. 100-fold increase in rate of
bacteremia
Immunization recommended. Most
effective when CD4+ > 200
73. HIV – respiratory disease - PcP
Single most common cause of pneumonia50% unaware of HIV
In 79% cd4+ < 100/ μL
Incidence near zero in cART + TMP-SMX
receiving patients
Symptoms – fever, dry cough, pleuritic
pain, indolent course
Extrapulmonary often seen (ophthalmic,
vasculitis, hematologic, renal)
74. HIV – respiratory disease - PcP
Most common finding on x-ray – normal(no perihilar infiltrates)
Laboratory of little value – elevated LDH,
increased A-a gradient
Diagnosis requires culture or PCR from BAL
Treatment – TMP-SMX (3 wks) – high rate of
adverse effects in HIV patients
Glucocorticoids if PaO2 < 70 or A-a > 35
Prophylaxis – in previously infected, CD4+
<200/15%, fever > 2 wks, candidiasis
75. HIV – respiratory disease - TB
Worldwide 33% of HIV associated deathDevelops early in the course of HIV
(median CD4+ of 330)
Clinical mostly typical pattern – fever,
night sweats, dyspnea, cavitary lesions in
upper lobes
Treatment involves risk of IRIS – should
apply preventive protocols (if cd4+ level
allows)
PPD > 5 mm, IGRA+ or close households –
considered latent – 9 m dual therapy
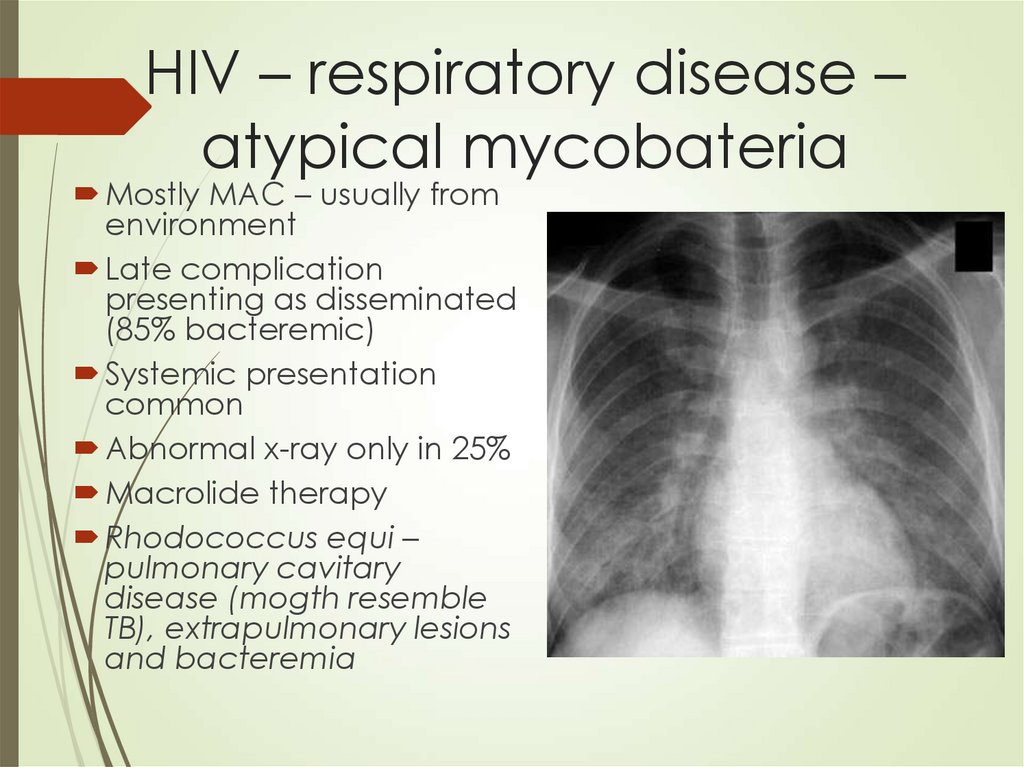
76. HIV – respiratory disease – atypical mycobateria
Mostly MAC – usually fromenvironment
Late complication
presenting as disseminated
(85% bacteremic)
Systemic presentation
common
Abnormal x-ray only in 25%
Macrolide therapy
Rhodococcus equi –
pulmonary cavitary
disease (mogth resemble
TB), extrapulmonary lesions
and bacteremia
77. HIV – respiratory disease - Fungal
HIV – respiratory disease FungalCryptococcal – fever, cough,
hemoptysis, abnormal x-ray (varying
pattern) and CNS involvement in
>90%
Aspergillus – not a common HIV
agent
Histoplasmossis – mostly as
dissemination
78. Pulmonary infections in cancer
Local factors – tumor, inflammation,obstruction, radiation
Systemic factors – metastatic
disease, chemotherapy
Differential diagnosis
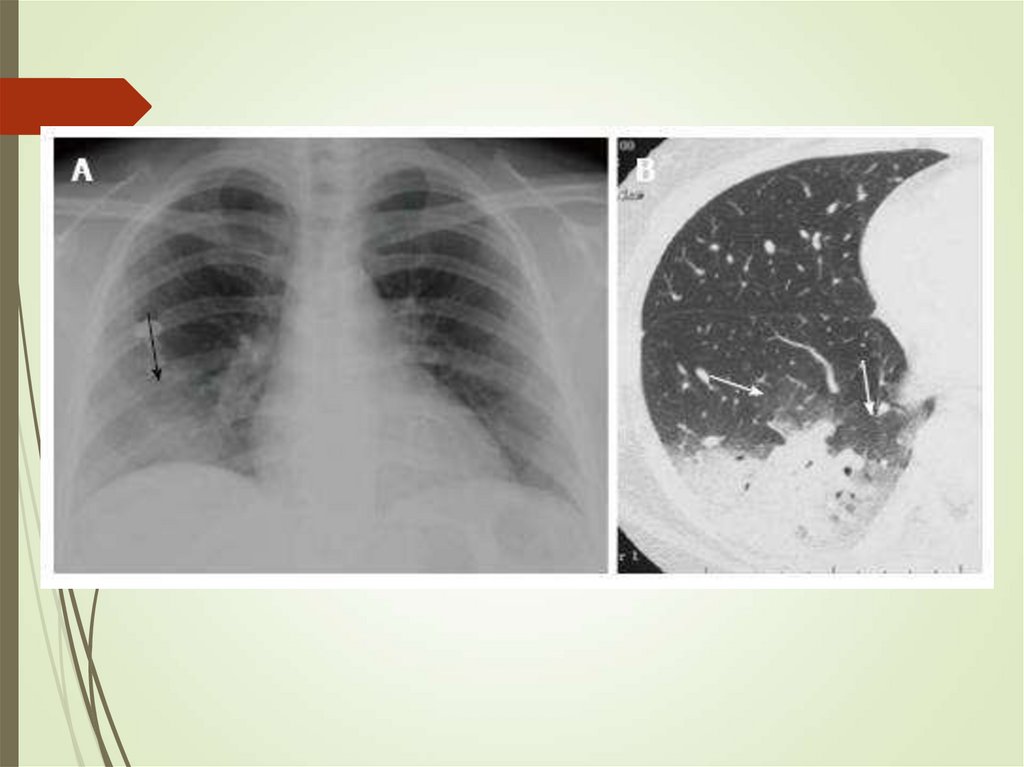
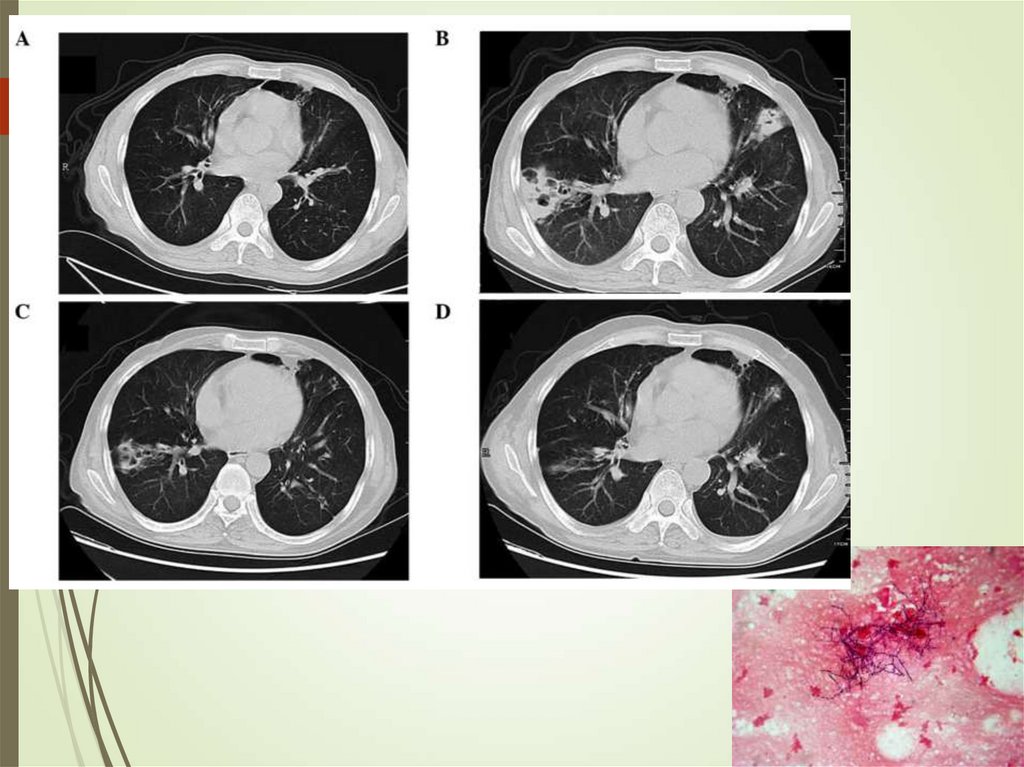
79. Pulmonary infections in cancer - Aspergillus
Can colonize skin and airwaysInvades lungs (IPA)
Diagnosis requires triad of host,
microbiologic and imaging evidence
Cough, pleuritic pain, hemoptysis
Culture, galactomannan (blood,
sputum)
Imaging – infiltrates, halo sign,
crescent























 Медицина
Медицина








